Machine learning predicts new anti-CRISPR proteins
- PMID: 32286628
- PMCID: PMC7229843
- DOI: 10.1093/nar/gkaa219
Machine learning predicts new anti-CRISPR proteins
Abstract
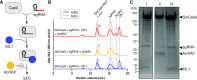
The increasing use of CRISPR-Cas9 in medicine, agriculture, and synthetic biology has accelerated the drive to discover new CRISPR-Cas inhibitors as potential mechanisms of control for gene editing applications. Many anti-CRISPRs have been found that inhibit the CRISPR-Cas adaptive immune system. However, comparing all currently known anti-CRISPRs does not reveal a shared set of properties for facile bioinformatic identification of new anti-CRISPR families. Here, we describe AcRanker, a machine learning based method to aid direct identification of new potential anti-CRISPRs using only protein sequence information. Using a training set of known anti-CRISPRs, we built a model based on XGBoost ranking. We then applied AcRanker to predict candidate anti-CRISPRs from predicted prophage regions within self-targeting bacterial genomes and discovered two previously unknown anti-CRISPRs: AcrllA20 (ML1) and AcrIIA21 (ML8). We show that AcrIIA20 strongly inhibits Streptococcus iniae Cas9 (SinCas9) and weakly inhibits Streptococcus pyogenes Cas9 (SpyCas9). We also show that AcrIIA21 inhibits SpyCas9, Streptococcus aureus Cas9 (SauCas9) and SinCas9 with low potency. The addition of AcRanker to the anti-CRISPR discovery toolkit allows researchers to directly rank potential anti-CRISPR candidate genes for increased speed in testing and validation of new anti-CRISPRs. A web server implementation for AcRanker is available online at http://acranker.pythonanywhere.com/.
© The Author(s) 2020. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures


References
-
- Bolotin A., Quinquis B., Sorokin A., Dusko Ehrlich S.. Clustered regularly interspaced short palindrome repeats (CRISPRs) have spacers of extrachromosomal origin. Microbiology. 2005; 151:2551–2561. - PubMed
-
- Horvath P., Barrangou R.. CRISPR/Cas, the immune system of bacteria and archaea. Science. 2010; 327:167–170. - PubMed
-
- Barrangou R. The roles of CRISPR–Cas systems in adaptive immunity and beyond. Curr. Opin. Immunol. 2015; 32:36–41. - PubMed
-
- Song G., Jia M., Chen K., Kong X., Khattak B., Xie C., Li A., Mao L.. CRISPR/Cas9: a powerful tool for crop genome editing. Crop J. 2016; 4:75–82.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

