Hydrosulfonylation of Alkenes with Sulfonyl Chlorides under Visible Light Activation
- PMID: 32286720
- PMCID: PMC7384135
- DOI: 10.1002/anie.202004070
Hydrosulfonylation of Alkenes with Sulfonyl Chlorides under Visible Light Activation
Abstract
Sulfonyl chlorides are inexpensive reactants extensively explored for functionalization, but never considered for radical hydrosulfonylation of alkenes. Herein, we report that tris(trimethylsilyl)silane is an ideal hydrogen atom donor enabling highly effective photoredox-catalyzed hydrosulfonylation of electron-deficient alkenes with sulfonyl chlorides. To increase the generality of this transformation, polarity-reversal catalysis (PRC) was successfully implemented for alkenes bearing alkyl substituents. This late-stage functionalization method tolerates a remarkably wide range of functional groups, is operationally simple, scalable, and allows access to building blocks which are important for medicinal chemistry and drug discovery.
Keywords: alkenes; hydrosulfonylation; photochemistry; radicals; sulfonyl chlorides.
© 2020 The Authors. Published by Wiley-VCH Verlag GmbH & Co. KGaA.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


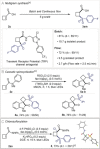
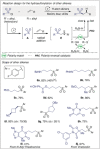


References
-
- None
-
- Patai S., Rappoport Z., Stirling C. J. M., The Chemistry of Sulphones and Sulfoxides, Wiley, New York, 1988;
-
- Ahmad I., Shagufta, Int. J. Pharmacol. Pharm. Sci. 2015, 7, 19–27;
-
- Scott K. A., Njardarson J. T., Top. Curr. Chem. 2018, 376, 5. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

