Highly Diastereoselective Functionalization of Piperidines by Photoredox-Catalyzed α-Amino C-H Arylation and Epimerization
- PMID: 32286827
- PMCID: PMC7318553
- DOI: 10.1021/jacs.9b13165
Highly Diastereoselective Functionalization of Piperidines by Photoredox-Catalyzed α-Amino C-H Arylation and Epimerization
Abstract
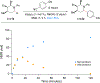
We report a photoredox-catalyzed α-amino C-H arylation reaction of highly substituted piperidine derivatives with electron-deficient cyano(hetero)arenes. The scope and limitations of the reaction were explored, with piperidines bearing multiple substitution patterns providing the arylated products in good yields and with high diastereoselectivity. To probe the mechanism of the overall transformation, optical and fluorescent spectroscopic methods were used to investigate the reaction. By employing flash-quench transient absorption spectroscopy, we were able to observe electron transfer processes associated with radical formation beyond the initial excited-state Ir(ppy)3 oxidation. Following the rapid and unselective C-H arylation reaction, a slower epimerization occurs to provide the high diastereomer ratio observed for a majority of the products. Several stereoisomerically pure products were resubjected to the reaction conditions, each of which converged to the experimentally observed diastereomer ratios. The observed distribution of diastereomers corresponds to a thermodynamic ratio of isomers based upon their calculated relative energies using density functional theory (DFT).
Conflict of interest statement
The authors declare no competing financial interest.
Figures



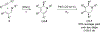

References
-
- Vitaku E; Smith DT; Njardarson JT Analysis of the Structural Diversity, Substitution Patterns, and Frequency of Nitrogen Heterocycles among U.S. FDA Approved Pharmaceuticals. J. Med. Chem. 2014, 57, 10257. - PubMed
- Typing the name of these drug and drug candidates into PubChem (http://pubchem.ncbi.nlm.nih.gov/) provides the compound structure, bioactivity, full list of published studies, and information regarding ongoing clinical trials, applications, and usage.
-
- Schultz DM; Yoon TP Solar Synthesis: Prospects in Visible Light Photocatalysis. Science 2014, 343, 1239176. - PMC - PubMed
- Prier CK; Rankic DA; MacMillan DWC Visible Light Photoredox Catalysis with Transition Metal Complexes: Applications in Organic Synthesis. Chem. Rev. 2013, 113, 5322. - PMC - PubMed
- Narayanam JMR; Stephenson CRJ Visible light photoredox catalysis: applications in organic synthesis. Chem. Soc. Rev. 2011, 40, 102. - PubMed
-
- Cho DW; Yoon UC; Mariano PS Studies Leading to the Development of a Single-Electron Transfer (SET) Photochemical Strategy for Syntheses of Macrocyclic Polyethers, Polythioethers, and Polyamides. Acc. Chem. Res. 2011, 44, 204. - PubMed
- Pandey G; Reddy GD; Kumaraswamy G Photoinduced Electron Transfer (PET) Promoted Cyclisations of 1-[N-alkyl-N-(trimethylsilyl)methyl] amines Tethered to Proximate Olefin: Mechanistic and Synthetic Perspectives. Tetrahedron 1994, 50, 8185.
- Yoon UC; Mariano PS Mechanistic and Synthetic Aspects of Amine-Enone Single Electron Transfer Photochemistry. Acc. Chem. Res. 1992, 25, 233.
- Pandey G; Kumaraswamy G; Bhalerao UT Photoinduced, S. E. T. Generation of α-amino radicals: A Practical Method for the Synthesis of Pyrrolidines and Piperidines. Tetrahedron Lett. 1989, 30, 6059.
-
- For other methods of α-amino arylation, see: Jiang H-J; Zhong X-M; Yu J; Zhang Y; Zhang X; Wu Y-D; Gong L-Z Assembling a Hybrid Pd Catalyst from a Chiral Anionic CoIII Complex and Ligand for Asymmetric C(sp3)–H Functionalization. Angew. Chem. Int. Ed. 2019, 58, 1803. - PubMed
- Greβies S; Klauck FJR; Kim JH; Daniliuc CG; Glorius F Ligand-Enabled Enantioselective Csp3–H Activation of Tetrahydroquinolines and Saturated Aza-Heterocycles by Rh1. Angew. Chem. Int. Ed. 2018, 57, 9950. - PubMed
- Jain P; Verma P; Xia G; Yu J-Q Enantioselective amine α-functionalization via palladium-catalysed C–H arylation of thioamides. Nat. Chem. 2017, 9, 140. - PMC - PubMed
- Spangler JE; Kobayashi Y; Verma P; Wang D-H; Yu J-Q α-Arylation of Saturated Azacycles and N-Methylamines via Palladium(II)-Catalyzed C(sp3)–H Coupling. J. Am. Chem. Soc. 2015, 137, 11876. - PMC - PubMed
- Peschiulli A; Smout V; Storr TE; Mitchell EA; Eliáš Z; Herrebout W; Berthelot D; Meerpoel L; Maes BUW Ruthenium-Catalyzed α-(Hetero)Arylation of Saturated Cyclic Amines: Reaction Scope and Mechanism. Chem. Eur. J. 2013,19, 10378. - PubMed
- Campos KR; Klapars A; Waldman JH; Dormer PG; Chen C-Y Enantioselective, Palladium-Catalyzed α-Arylation of N-Boc-pyrrolidine. J. Am. Chem. Soc. 2006, 128, 3538. - PubMed
- Pastine SJ; Gribkov DV; Sames D sp3 C–H Bond Arylation Directed by Amidine Protecting Group: a-Arylation of Pyrrolidines and Piperidines. J. Am. Chem. Soc. 2006, 128, 14220. - PubMed
- Li Z; Li C-J CuBr-Catalyzed Direct Indolation of Tetrahydroisoquinolines via Cross-Dehydrogenative Coupling between sp3 C–H and sp2 C–H Bonds. J. Am. Chem. Soc. 2005, 127, 6968. - PubMed

