CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial
- PMID: 32286905
- PMCID: PMC7280047
- DOI: 10.1200/JCO.19.03279
CD4/CD8 T-Cell Selection Affects Chimeric Antigen Receptor (CAR) T-Cell Potency and Toxicity: Updated Results From a Phase I Anti-CD22 CAR T-Cell Trial
Abstract
Purpose: Patients with B-cell acute lymphoblastic leukemia who experience relapse after or are resistant to CD19-targeted immunotherapies have limited treatment options. Targeting CD22, an alternative B-cell antigen, represents an alternate strategy. We report outcomes on the largest patient cohort treated with CD22 chimeric antigen receptor (CAR) T cells.
Patients and methods: We conducted a single-center, phase I, 3 + 3 dose-escalation trial with a large expansion cohort that tested CD22-targeted CAR T cells for children and young adults with relapsed/refractory CD22+ malignancies. Primary objectives were to assess the safety, toxicity, and feasibility. Secondary objectives included efficacy, CD22 CAR T-cell persistence, and cytokine profiling.
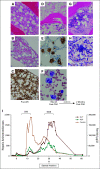
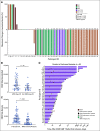
Results: Fifty-eight participants were infused; 51 (87.9%) after prior CD19-targeted therapy. Cytokine release syndrome occurred in 50 participants (86.2%) and was grade 1-2 in 45 (90%). Symptoms of neurotoxicity were minimal and transient. Hemophagocytic lymphohistiocytosis-like manifestations were seen in 19/58 (32.8%) of subjects, prompting utilization of anakinra. CD4/CD8 T-cell selection of the apheresis product improved CAR T-cell manufacturing feasibility as well as heightened inflammatory toxicities, leading to dose de-escalation. The complete remission rate was 70%. The median overall survival was 13.4 months (95% CI, 7.7 to 20.3 months). Among those who achieved a complete response, the median relapse-free survival was 6.0 months (95% CI, 4.1 to 6.5 months). Thirteen participants proceeded to stem-cell transplantation.
Conclusion: In the largest experience of CD22 CAR T-cells to our knowledge, we provide novel information on the impact of manufacturing changes on clinical outcomes and report on unique CD22 CAR T-cell toxicities and toxicity mitigation strategies. The remission induction rate supports further development of CD22 CAR T cells as a therapeutic option in patients resistant to CD19-targeted immunotherapy.
Trial registration: ClinicalTrials.gov NCT02315612.
Figures




Comment in
-
Anti-CD22 CAR T cells in ALL.Nat Rev Clin Oncol. 2020 Jul;17(7):391. doi: 10.1038/s41571-020-0379-x. Nat Rev Clin Oncol. 2020. PMID: 32358575 No abstract available.
References
-
- Jen EY, Xu Q, Schetter A, et al: FDA approval: Blinatumomab for patients with B-cell precursor acute lymphoblastic leukemia in morphologic remission with minimal residual disease. Clin Cancer Res, 25:473-477, 2019. - PubMed
-
- Przepiorka D, Ko CW, Deisseroth A, et al. FDA approval: Blinatumomab. Clin Cancer Res. 2015;21:4035–4039. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

