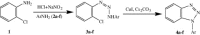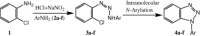Facile route for N 1-aryl benzotriazoles from diazoamino arynes via CuI-mediated intramolecular N-arylation
- PMID: 32287442
- PMCID: PMC7111836
- DOI: 10.1016/j.tetlet.2010.08.083
Facile route for N 1-aryl benzotriazoles from diazoamino arynes via CuI-mediated intramolecular N-arylation
Abstract
A facile and high-yielding protocol for diverse benzotriazoles through intramolecular N-arylation of different o-chloro-1,2,3-benzotriazenes using CuI/Cs2CO3 has been developed.
Keywords: Amines; Benzotriazole; CuI; Diazonium salt; N-Arylation.
Copyright © 2010 Elsevier Ltd. All rights reserved.
Figures
References
-
- Carlini R., Bria E., Giannarelli D., Ferretti G., Felici A., Papaldo P., Fabi A., Nistico C., Cosimo S. Di.; Ruggeri, E. M.; Milella, M.; Mottolese, M.; Terzoli, E.; Cognetti, F. Cancer. 2005;104:1335–1557. - PubMed
- Semple G., Skinner P.J., Cherrier M.C., Webb P.J., Sage C.R., Tamura S.Y., Chen R., Richman J.G., Connolly D.T. J. Med. Chem. 2006;49:1227–1230. - PubMed
-
- Dipesa A.J., Donahue K.M., Dombroski M.A., Elliott N.C., Gabel C.A., Han S., Hynes T.R., LeMotte P.K., Mansour M.N., Marr E.S., Letavic M.A., Pandit J., Ripin D.B., Sweeney F.J., Tan D., Tao Y. J. Med. Chem. 2005;48:5728–5737. - PubMed
- Wu C.Y., King K.Y., Fang J.M., Wu Y.T., Ho M.Y., Liao C.L., Shie J., Liang P.H., Wong C.H. Chem. Biol. 2006;13:261–268. - PMC - PubMed
- Katritzky A.R., Jiang J., Urogdi L. Tetrahedron Lett. 1989;25:3303–3306.
- Wender P.A., Touami S.M., Alayrac C., Philipp U.C. J. Am. Chem. Soc. 1996;118:6522–6523.
- Caliendo G., Greco G., Grieco P., Novellino E., Perissuttil E., Santagada V., Barbarul D., Esposit E., De Blas A. Eur. J. Med. Chem. 1996;31:207–213.
-
- Katritzky A.R., Lan X., Jason Z., Yang Z., Denisko O.V. Chem. Rev. 1998;98:409–548. - PubMed
- Katritzky A.R., Manju K., Singh S.K., Meher N.K. Tetrahedron. 2005;61:2555–2581.
- Katritzky A.R., Rogovoy B.V. Chem. Eur. J. 2003;19:4586–4593. - PubMed
- Katritzky A.R., Lan X. Chem. Soc. Rev. 1994;23:363–373.
- Katritzky A.R., Henderson S.A., Yang B. J. Heterocycl. Chem. 1998;35:1123–1159.
-
- Verma A.K., Kesharwani T., Singh J., Tandon V., Larock R.C. Angew. Chem., Int. Ed. 2009;48:1138–1143. - PubMed
- Kale R.R., Prasad V., Mohapatra P.P., Tiwari V.K. Monatsh. Chem. 2010
-
- Reynolds G.A. J. Org. Chem. 1964;29:3733–3734.
LinkOut - more resources
Full Text Sources