Diagnostic tools for tackling febrile illness and enhancing patient management
- PMID: 32287568
- PMCID: PMC7114275
- DOI: 10.1016/j.mee.2018.10.001
Diagnostic tools for tackling febrile illness and enhancing patient management
Abstract
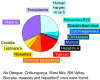
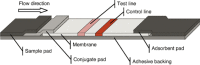
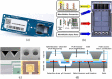
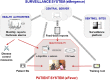
Most patients with acute infectious diseases develop fever, which is frequently a reason to visit health facilities in resource-limited settings. The symptomatic overlap between febrile diseases impedes their diagnosis on clinical grounds. Therefore, the World Health Organization promotes an integrated management of febrile illness. Along this line, we present an overview of endemic and epidemic etiologies of fever and state-of-the-art diagnostic tools used in the field. It becomes evident that there is an urgent need for the development of novel technologies to fulfill end-users' requirements. This need can be met with point-of-care and near-patient diagnostic platforms, as well as e-Health clinical algorithms, which co-assess test results with key clinical elements and biosensors, assisting clinicians in patient triage and management, thus enhancing disease surveillance and outbreak alerts. This review gives an overview of diagnostic technologies featuring a platform based approach: (i) assay (nucleic acid amplification technologies are examined); (ii) cartridge (microfluidic technologies are presented); (iii) instrument (various detection technologies are discussed); and at the end proposes a way that such technologies can be interfaced with electronic clinical decision-making algorithms towards a broad and complete diagnostic ecosystem.
Keywords: Disease surveillance; Electronic clinical decision algorithms; Febrile illness; Microfluidics; Nucleic acid amplification; Point-of-care diagnostics.
© 2018 Elsevier B.V. All rights reserved.
Conflict of interest statement
Conflicts of interest: none.
Figures























References
-
- Liu L., Johnson H.L., Cousens S., Perin J., Scott S., Lawn J.E. Global, regional, and national causes of child mortality: an updated systematic analysis for 2010 with time trends since 2000. Lancet. 2012;379:2151–2161. - PubMed
-
- WHO . World Health Organization; Geneva, Switzerland: 2013. WHO Informal Consultation on Fever Management in Peripheral Health Care Settings: A Global Review of Evidence and Practice.http://www.who.int/malaria/publications/atoz/9789241506489/en/ Available at:
-
- Crump J.A. Typhoid fever and the challenge of nonmalaria febrile illness in Sub-Saharan Africa. Clin. Infect. Dis. 2012;54(8):1107–1109. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
