Virus-like particle-based vaccines for animal viral infections
- PMID: 32287712
- PMCID: PMC7115488
- DOI: 10.1016/j.inmuno.2012.08.002
Virus-like particle-based vaccines for animal viral infections
Abstract
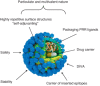
Vaccination is considered one of the most effective ways to control pathogens and prevent diseases in humans as well as in the veterinary field. Traditional vaccines against animal viral diseases are based on inactivated or attenuated viruses, but new subunit vaccines are gaining attention from researchers in animal vaccinology. Among these, virus-like particles (VLPs) represent one of the most appealing approaches opening up interesting frontiers in animal vaccines. VLPs are robust protein scaffolds exhibiting well-defined geometry and uniformity that mimic the overall structure of the native virions but lack the viral genome. They are often antigenically indistinguishable from the virus from which they were derived and present important advantages in terms of safety. VLPs can stimulate strong humoral and cellular immune responses and have been shown to exhibit self-adjuvanting abilities. In addition to their suitability as a vaccine for the homologous virus from which they are derived, VLPs can also be used as vectors for the multimeric presentation of foreign antigens. VLPs have therefore shown dramatic effectiveness as candidate vaccines; nevertheless, only one veterinary VLP-base vaccine is licensed. Here, we review and examine in detail the current status of VLPs as a vaccine strategy in the veterinary field, and discuss the potential advantages and challenges of this technology.
La vacunación constituye uno de los procedimientos más eficaces para controlar los patógenos y prevenir enfermedades tanto en seres humanos como en el campo veterinario. Las vacunas tradicionales frente a enfermedades animales se basan por lo general en la utilización de virus atenuados o inactivados. Sin embargo, las vacunas de subunidad están ganando terreno progresivamente en el campo de la sanidad animal. Entre ellas, las vacunas basadas en pseudopartículas virales o VLPs (por su nombre en inglés virus-like particles), representan una de las estrategias más atractivas actualmente en el campo de las vacunas para animales. Las VLPs son estructuras proteicas con una geometría y uniformidad muy definidas, que mimetizan la estructura de los virus nativos pero carecen de genoma viral. Por lo general son antigénicamente indistinguibles de los virus de los que proceden y su empleo como inmunógenos presenta importantes ventajas en términos de seguridad. Las VLPs pueden inducir una fuerte respuesta inmune, tanto humoral como celular, y se ha demostrado que poseen capacidad de actuar como adyuvantes (self-adjuvanting). Además de su idoneidad como vacunas frente al virus homólogo del cual proceden, las VLPs también se pueden utilizar como vectores para la presentación multimérica de antígenos heterólogos. Las VLPs han mostrado una elevada eficacia como candidatos vacunales, sin embargo, hasta el momento sólo una vacuna basada en VLPs ha sido autorizada y comercializada en el campo veterinario. En este trabajo se revisa el estado actual de las VLP empleadas como nuevas estrategias vacunales en el campo de la veterinaria, analizando las potenciales ventajas y desafíos que enfrenta esta tecnología.
Keywords: Animal vaccines; Vaccine vectors; Virus-like particles.
Copyright © 2012 Sociedad Española de Inmunología. Published by Elsevier España, S.L. All rights reserved.
Figures
Similar articles
-
Virus-like particles: the new frontier of vaccines for animal viral infections.Vet Immunol Immunopathol. 2012 Aug 15;148(3-4):211-25. doi: 10.1016/j.vetimm.2012.04.026. Epub 2012 Jun 1. Vet Immunol Immunopathol. 2012. PMID: 22705417 Free PMC article. Review.
-
Designing Chimeric Virus-like Particle-based Vaccines for Human Papillomavirus and HIV: Lessons Learned.AIDS Rev. 2019;21(4):218-232. doi: 10.24875/AIDSRev.19000114. AIDS Rev. 2019. PMID: 31834327
-
Virus-like particles: potential veterinary vaccine immunogens.Res Vet Sci. 2012 Oct;93(2):553-9. doi: 10.1016/j.rvsc.2011.10.018. Epub 2011 Nov 17. Res Vet Sci. 2012. PMID: 22100244 Review.
-
Design of novel vaccines based on virus-like particles or chimeric virions.Subcell Biochem. 2013;68:631-65. doi: 10.1007/978-94-007-6552-8_21. Subcell Biochem. 2013. PMID: 23737067 Review.
-
Virus-like Particle Vaccines and Platforms for Vaccine Development.Viruses. 2023 May 2;15(5):1109. doi: 10.3390/v15051109. Viruses. 2023. PMID: 37243195 Free PMC article. Review.
Cited by
-
The Coming Age of Insect Cells for Manufacturing and Development of Protein Therapeutics.Ind Eng Chem Res. 2018 Aug 8;57(31):10061-10070. doi: 10.1021/acs.iecr.8b00985. Epub 2018 Jul 9. Ind Eng Chem Res. 2018. PMID: 30886455 Free PMC article.
-
Virus-like particles in poultry disease: an approach to effective and safe vaccination.Front Vet Sci. 2024 Sep 9;11:1405605. doi: 10.3389/fvets.2024.1405605. eCollection 2024. Front Vet Sci. 2024. PMID: 39315089 Free PMC article. Review.
-
Chimeric VLPs Based on HIV-1 Gag and a Fusion Rabies Glycoprotein Induce Specific Antibodies against Rabies and Foot-and-Mouth Disease Virus.Vaccines (Basel). 2021 Mar 12;9(3):251. doi: 10.3390/vaccines9030251. Vaccines (Basel). 2021. PMID: 33809060 Free PMC article.
-
Torque Teno Virus: Lights and Shades.Viruses. 2025 Feb 27;17(3):334. doi: 10.3390/v17030334. Viruses. 2025. PMID: 40143262 Free PMC article. Review.
-
Recent Development of Ruminant Vaccine Against Viral Diseases.Front Vet Sci. 2021 Nov 3;8:697194. doi: 10.3389/fvets.2021.697194. eCollection 2021. Front Vet Sci. 2021. PMID: 34805327 Free PMC article. Review.
References
-
- Jennings G.T., Bachmann M.F. The coming of age of virus-like particle vaccines. Biol Chem. 2008;389:521–536. - PubMed
-
- Liu F., Ge S., Li L., Wu X., Liu Z., Wang Z. Virus-like particles: potential veterinary vaccine immunogens. Res Vet Sci. 2012;93:553–559. - PubMed
-
- D’Aoust M.A., Couture M.M., Charland N., Trepanier S., Landry N., Ors F. The production of hemagglutinin-based virus-like particles in plants: a rapid, efficient and safe response to pandemic influenza. Plant Biotechnol J. 2010;8:607–619. - PubMed
-
- Bachmann M.F., Jennings G.T. Vaccine delivery: a matter of size, geometry, kinetics and molecular patterns. Nat Rev Immunol. 2010;10:787–796. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
