Nek2-mediated GAS2L1 phosphorylation and centrosome-linker disassembly induce centrosome disjunction
- PMID: 32289147
- PMCID: PMC7199859
- DOI: 10.1083/jcb.201909094
Nek2-mediated GAS2L1 phosphorylation and centrosome-linker disassembly induce centrosome disjunction
Abstract
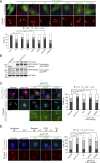
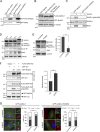
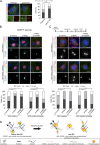
Centrosome disjunction occurs in late G2 to facilitate bipolar spindle formation and is mediated by the NIMA-related kinase Nek2. Here, we show that GAS2L1, a microtubule- and F-actin-binding protein required for centrosome disjunction, undergoes Nek2-mediated phosphorylation at Ser352 in G2/M. The phosphorylation is essential for centrosome disjunction in late G2 and for proper spindle assembly and faithful chromosome segregation in mitosis. GAS2L1 contains a calponin-homology (CH) domain and a GAS2-related (GAR) domain, which bind to F-actin and microtubules, respectively. Notably, the CH and GAR domains bind to each other to inhibit the functions of both domains, and Ser352 phosphorylation disrupts the interaction between the two domains and relieves the autoinhibition. We dissected the roles of the GAS2L1 phosphorylation and of centrosome-linker disassembly, which is another Nek2-mediated event, and found that these events together trigger centrosome disjunction. Therefore, our findings demonstrate the concerted Nek2 actions that split the centrosomes in late G2.
© 2020 Au et al.
Figures










References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

