Endothelial mTOR maintains hematopoiesis during aging
- PMID: 32289154
- PMCID: PMC7971143
- DOI: 10.1084/jem.20191212
Endothelial mTOR maintains hematopoiesis during aging
Abstract
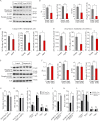
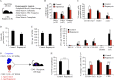
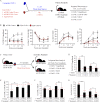
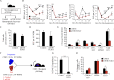
Aging leads to a decline in hematopoietic stem and progenitor cell (HSPC) function. We recently discovered that aging of bone marrow endothelial cells (BMECs) leads to an altered crosstalk between the BMEC niche and HSPCs, which instructs young HSPCs to behave as aged HSPCs. Here, we demonstrate aging leads to a decrease in mTOR signaling within BMECs that potentially underlies the age-related impairment of their niche activity. Our findings reveal that pharmacological inhibition of mTOR using Rapamycin has deleterious effects on hematopoiesis. To formally determine whether endothelial-specific inhibition of mTOR can influence hematopoietic aging, we conditionally deleted mTOR in ECs (mTOR(ECKO)) of young mice and observed that their HSPCs displayed attributes of an aged hematopoietic system. Transcriptional profiling of HSPCs from mTOR(ECKO) mice revealed that their transcriptome resembled aged HSPCs. Notably, during serial transplantations, exposure of wild-type HSPCs to an mTOR(ECKO) microenvironment was sufficient to recapitulate aging-associated phenotypes, confirming the instructive role of EC-derived signals in governing HSPC aging.
© 2020 Ramalingam et al.
Conflict of interest statement
Disclosures: The authors declare no competing interests exist.
Figures



References
-
- Aranda, J.F., Reglero-Real N., Marcos-Ramiro B., Ruiz-Sáenz A., Fernández-Martín L., Bernabé-Rubio M., Kremer L., Ridley A.J., Correas I., Alonso M.A., and Millán J.. 2013. MYADM controls endothelial barrier function through ERM-dependent regulation of ICAM-1 expression. Mol. Biol. Cell. 24:483–494. 10.1091/mbc.e11-11-0914 - DOI - PMC - PubMed
-
- Armand, P., Kim H.T., Sainvil M.M., Lange P.B., Giardino A.A., Bachanova V., Devine S.M., Waller E.K., Jagirdar N., Herrera A.F., et al. . 2016. The addition of sirolimus to the graft-versus-host disease prophylaxis regimen in reduced intensity allogeneic stem cell transplantation for lymphoma: a multicentre randomized trial. Br. J. Haematol. 173:96–104. 10.1111/bjh.13931 - DOI - PMC - PubMed
-
- Balducci, L. 2003. Myelosuppression and its consequences in elderly patients with cancer. Oncology (Williston Park). 17(11, Suppl 11):27–32. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

