Blinatumomab consolidation and maintenance therapy in adults with relapsed/refractory B-precursor acute lymphoblastic leukemia
- PMID: 32289160
- PMCID: PMC7160264
- DOI: 10.1182/bloodadvances.2019000874
Blinatumomab consolidation and maintenance therapy in adults with relapsed/refractory B-precursor acute lymphoblastic leukemia
Abstract
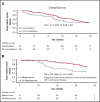
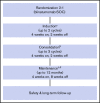
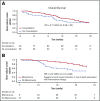
In a phase 3 clinical study of heavily pretreated adults with relapsed/refractory (R/R) acute lymphoblastic leukemia (ALL), overall survival (OS) following blinatumomab, a BiTE (bispecific T-cell engager) immunooncology therapy, was significantly improved vs chemotherapy following induction (cycles 1 to 2). Here we report the efficacy and safety of those who received additional cycles of blinatumomab. Blinatumomab was administered as a continuous IV infusion for 4 weeks in a 6-week cycle. Patients who achieved a bone marrow response (≤5% blasts) or complete remission (full, partial, or incomplete hematological recovery) during induction could receive additional cycles of blinatumomab. OS and relapse-free survival (RFS) for consolidation (cycles 3 to 5) vs no consolidation, and maintenance (cycles ≥6) vs no maintenance were analyzed using Simon-Makuch and Mantel-Byar odds ratios. Of 267 patients who received blinatumomab induction, 86 (32%) entered consolidation and 36 (13%) entered maintenance. Evidence of longer OS was demonstrated among the maintenance group compared with no-maintenance (median OS [95% confidence interval, CI]: not reached for maintenance vs 15.5 months for no maintenance). Median RFS (months; 95% CI) was numerically longer among maintenance group (14.5; 7.1 to 21.9) compared with no-maintenance (9.8; 8.5 to 11.1). A lower incidence of adverse events was seen during maintenance (72.2%) compared with induction (97.2%) and consolidation (86.1%). Adults with R/R ALL who achieved remission following blinatumomab induction had longer survival on continuation therapy than those who discontinued blinatumomab early, supporting the use of blinatumomab as long-term therapy. No new safety signals were reported. This trial was registered at www.clinicaltrials.gov as #NCT02013167.
© 2020 by The American Society of Hematology.
Conflict of interest statement
Conflict-of-interest disclosure: A.R. received consulting/advisory fees from Amgen, Novartis, Roche/Genentech, and Italfarmaco, and travel expenses from Celgene and Sanofi. F.H. received honoraria and consulting/advisory fees from Amgen, Bristol-Myers Squibb, Novartis, Incyte, Jazz Pharma, and Pfizer. P.C. received travel support from Amgen. Q.T. is employed by and holds stock in Amgen Inc. J.F. is employed by and holds stock in Amgen Inc. M.S.T. received honoraria and speakers’ bureau fees from Amgen Inc; consulting/advisory fees from Amgen Inc, Roche, and Regeneron; and travel expenses from Amgen Inc and Roche. P.Z. declares no competing financial interests.
Figures


References
-
- Phelan KW, Advani AS. Novel therapies in acute lymphoblastic leukemia. Curr Hematol Malig Rep. 2018;13(4):289-299. - PubMed
-
- BLINCYTO® (blinatumomab) package insert. Thousand Oaks, CA: Amgen Inc; 2018.

