Histologic safety of transcranial focused ultrasound neuromodulation and magnetic resonance acoustic radiation force imaging in rhesus macaques and sheep
- PMID: 32289711
- PMCID: PMC7196031
- DOI: 10.1016/j.brs.2020.02.017
Histologic safety of transcranial focused ultrasound neuromodulation and magnetic resonance acoustic radiation force imaging in rhesus macaques and sheep
Abstract
Background: Neuromodulation by transcranial focused ultrasound (FUS) offers the potential to non-invasively treat specific brain regions, with treatment location verified by magnetic resonance acoustic radiation force imaging (MR-ARFI).
Objective: To investigate the safety of these methods prior to widespread clinical use, we report histologic findings in two large animal models following FUS neuromodulation and MR-ARFI.
Methods: Two rhesus macaques and thirteen Dorset sheep were studied. FUS neuromodulation was targeted to the primary visual cortex in rhesus macaques and to subcortical locations, verified by MR-ARFI, in eleven sheep. Both rhesus macaques and five sheep received a single FUS session, whereas six sheep received repeated sessions three to six days apart. The remaining two control sheep did not receive ultrasound but otherwise underwent the same anesthetic and MRI procedures as the eleven experimental sheep. Hematoxylin and eosin-stained sections of brain tissue (harvested zero to eleven days following FUS) were evaluated for tissue damage at FUS and control locations as well as tissue within the path of the FUS beam. TUNEL staining was used to evaluate for the presence of apoptosis in sheep receiving high dose FUS.
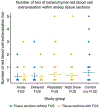
Results: No FUS-related pre-mortem histologic findings were observed in the rhesus macaques or in any of the examined sheep. Extravascular red blood cells (RBCs) were present within the meninges of all sheep, regardless of treatment group. Similarly, small aggregates of perivascular RBCs were rarely noted in non-target regions of neural parenchyma of FUS-treated (8/11) and untreated (2/2) sheep. However, no concurrent histologic abnormalities were observed, consistent with RBC extravasation occurring as post-mortem artifact following brain extraction. Sheep within the high dose FUS group were TUNEL-negative at the targeted site of FUS.
Conclusions: The absence of FUS-related histologic findings suggests that the neuromodulation and MR-ARFI protocols evaluated do not cause tissue damage.
Keywords: Focused ultrasound; Magnetic resonance acoustic radiation force imaging; Neuromodulation; Safety.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Figures








Similar articles
-
Ultrasound focusing using magnetic resonance acoustic radiation force imaging: application to ultrasound transcranial therapy.Med Phys. 2010 Jun;37(6):2934-42. doi: 10.1118/1.3395553. Med Phys. 2010. PMID: 20632605
-
On the accuracy of optically tracked transducers for image-guided transcranial ultrasound.Int J Comput Assist Radiol Surg. 2019 Aug;14(8):1317-1327. doi: 10.1007/s11548-019-01988-0. Epub 2019 May 8. Int J Comput Assist Radiol Surg. 2019. PMID: 31069643 Free PMC article.
-
Considerations for ultrasound exposure during transcranial MR acoustic radiation force imaging.Sci Rep. 2019 Nov 7;9(1):16235. doi: 10.1038/s41598-019-52443-8. Sci Rep. 2019. PMID: 31700021 Free PMC article.
-
Neuromodulation with transcranial focused ultrasound.Neurosurg Focus. 2018 Feb;44(2):E14. doi: 10.3171/2017.11.FOCUS17621. Neurosurg Focus. 2018. PMID: 29385924 Free PMC article. Review.
-
Unveiling the potential of ultrasound in brain imaging: Innovations, challenges, and prospects.Ultrasonics. 2025 Jan;145:107465. doi: 10.1016/j.ultras.2024.107465. Epub 2024 Sep 12. Ultrasonics. 2025. PMID: 39305556 Review.
Cited by
-
Safety of Clinical Ultrasound Neuromodulation.Brain Sci. 2022 Sep 22;12(10):1277. doi: 10.3390/brainsci12101277. Brain Sci. 2022. PMID: 36291211 Free PMC article. Review.
-
Ultrasonic Deep Brain Neuromodulation in Acute Disorders of Consciousness: A Proof-of-Concept.Brain Sci. 2022 Mar 23;12(4):428. doi: 10.3390/brainsci12040428. Brain Sci. 2022. PMID: 35447960 Free PMC article.
-
Neuromodulation with Ultrasound: Hypotheses on the Directionality of Effects and Community Resource.medRxiv [Preprint]. 2025 Feb 21:2024.06.14.24308829. doi: 10.1101/2024.06.14.24308829. medRxiv. 2025. PMID: 38947047 Free PMC article. Preprint.
-
Mathematical Model of Ultrasound Attenuation With Skull Thickness for Transcranial-Focused Ultrasound.Front Neurosci. 2022 Feb 17;15:778616. doi: 10.3389/fnins.2021.778616. eCollection 2021. Front Neurosci. 2022. PMID: 35250434 Free PMC article.
-
Practical Targeting Errors During Optically Tracked Transcranial Focused Ultrasound Using MR-ARFI and Array- Based Steering.IEEE Trans Biomed Eng. 2024 Sep;71(9):2740-2748. doi: 10.1109/TBME.2024.3391383. Epub 2024 Aug 21. IEEE Trans Biomed Eng. 2024. PMID: 38640051 Free PMC article.
References
-
- Attali David, Houdouin Alexandre, Tanter Mickael, and Aubry Jean-François. 2019. “Thermal Safety of Transcranial Focused Simulation in Human: Retrospective Numerical Estimation of Thermal Rise in Cortical and Subcortical Simulation Setups.” In 19th International Symposium on Therapeutic Ultrasound, Barcelona.
-
- Auboiroux Vincent, Viallon Magalie, Roland Joerg, Hyacinthe Jean-Noël, Petrusca Lorena, Morel Denis R, Goget Thomas, et al. 2012. “ARFI-Prepared MRgHIFU in Liver: Simultaneous Mapping of ARFI-Displacement and Temperature Elevation, Using a Fast GRE-EPI Sequence.” Magnetic Resonance in Medicine 68 (3): 932–46. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

