Transcriptional Profiling of the Adult Hair Follicle Mesenchyme Reveals R-spondin as a Novel Regulator of Dermal Progenitor Function
- PMID: 32289736
- PMCID: PMC7155209
- DOI: 10.1016/j.isci.2020.101019
Transcriptional Profiling of the Adult Hair Follicle Mesenchyme Reveals R-spondin as a Novel Regulator of Dermal Progenitor Function
Abstract
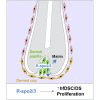
The adult hair follicle (HF) undergoes successive regeneration driven by resident epithelial stem cells and neighboring mesenchyme. Recent work described the existence of HF dermal stem cells (hfDSCs), but the genetic regulation of hfDSCs and their daughter cell lineages in HF regeneration remains unknown. Here we prospectively isolate functionally distinct mesenchymal compartment in the HF (dermal cup [DC; includes hfDSCs] and dermal papilla) and define the transcriptional programs involved in hfDSC function and acquisition of divergent mesenchymal fates. From this, we demonstrate cross-compartment mesenchymal signaling within the HF niche, whereby DP-derived R-spondins act to stimulate proliferation of both hfDSCs and epithelial progenitors during HF regeneration. Our findings describe unique transcriptional programs that underlie the functional heterogeneity among specialized fibroblasts within the adult HF and identify a novel regulator of mesenchymal progenitor function during tissue regeneration.
Keywords: Biological Sciences; Developmental Biology; Stem Cells Research.
Copyright © 2020 The Author(s). Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Declaration of Interests The authors declare no competing interests.
Figures




References
-
- Abo A., Clevers H. Modulating WNT receptor turnover for tissue repair. Nat. Biotechnol. 2012;30:835–836. - PubMed
-
- Barker N., Huch M., Kujala P., van de Wetering M., Snippert H.J., van Es J.H., Sato T., Stange D.E., Begthel H., van den Born M. Lgr5(+ve) stem cells drive self-renewal in the stomach and build long-lived gastric units in vitro. Cell Stem Cell. 2010;6:25–36. - PubMed
-
- Barker N., van Es J.H., Kuipers J., Kujala P., van den Born M., Cozijnsen M., Haegebarth A., Korving J., Begthel H., Peters P.J. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. - PubMed
-
- Bodo E., Kromminga A., Biro T., Borbiro I., Gaspar E., Zmijewski M.A., van Beek N., Langbein L., Slominski A.T., Paus R. Human female hair follicles are a direct, nonclassical target for thyroid-stimulating hormone. J. Invest. Dermatol. 2009;129:1126–1139. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

