Comprehensive History of CSP Genes: Evolution, Phylogenetic Distribution and Functions
- PMID: 32290210
- PMCID: PMC7230875
- DOI: 10.3390/genes11040413
Comprehensive History of CSP Genes: Evolution, Phylogenetic Distribution and Functions
Abstract
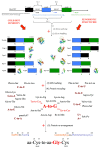
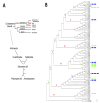
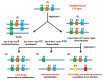
In this review we present the developmental, histological, evolutionary and functional properties of insect chemosensory proteins (CSPs) in insect species. CSPs are small globular proteins folded like a prism and notoriously known for their complex and arguably obscure function(s), particularly in pheromone olfaction. Here, we focus on direct functional consequences on protein function depending on duplication, expression and RNA editing. The result of our analysis is important for understanding the significance of RNA-editing on functionality of CSP genes, particularly in the brain tissue.
Keywords: RNA mutation; adaptive process; lipid transport; neuroplasticity; tandem duplication; xenobiotic resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Nomura A., Kawasaki K., Kubo T., Natori S. Purification and localization of p10, a novel protein that increases in nymphal regenerating legs of Periplaneta americana (American cockroach) Int. J. Dev. Biol. 1992;36:391–398. - PubMed
-
- Picimbon J.F., Leal W.S. Olfactory soluble proteins of cockroaches. Insect Biochem. Mol. Biol. 1999;30:973–978. doi: 10.1016/S0965-1748(99)00073-9. - DOI
-
- Angeli S., Ceron F., Scaloni A., Monti M., Monteforti G., Minnocci A., Petacchi R., Pelosi P. Purification, structural characterization, cloning and immunocytochemical localization of chemoreception proteins from Schistocerca gregaria. Eur. J. Biochem. 1999;262:745–754. doi: 10.1046/j.1432-1327.1999.00438.x. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

