BCL-2 Proteins in Pathogenesis and Therapy of B-Cell Non-Hodgkin Lymphomas
- PMID: 32290241
- PMCID: PMC7226356
- DOI: 10.3390/cancers12040938
BCL-2 Proteins in Pathogenesis and Therapy of B-Cell Non-Hodgkin Lymphomas
Abstract
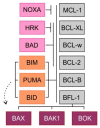
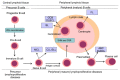
The ability to inhibit mitochondrial apoptosis is a hallmark of B-cell non-Hodgkin lymphomas (B-NHL). Activation of mitochondrial apoptosis is tightly controlled by members of B-cell leukemia/lymphoma-2 (BCL-2) family proteins via protein-protein interactions. Altering the balance between anti-apoptotic and pro-apoptotic BCL-2 proteins leads to apoptosis evasion and extended survival of malignant cells. The pro-survival BCL-2 proteins: B-cell leukemia/lymphoma-2 (BCL-2/BCL2), myeloid cell leukemia-1 (MCL-1/MCL1) and B-cell lymphoma-extra large (BCL-XL/BCL2L1) are frequently (over)expressed in B-NHL, which plays a crucial role in lymphoma pathogenesis, disease progression, and drug resistance. The efforts to develop inhibitors of anti-apoptotic BCL-2 proteins have been underway for several decades and molecules targeting anti-apoptotic BCL-2 proteins are in various stages of clinical testing. Venetoclax is a highly specific BCL-2 inhibitor, which has been approved by the US Food and Drug Agency (FDA) for the treatment of patients with chronic lymphocytic leukemia (CLL) and is in advanced clinical testing in other types of B-NHL. In this review, we summarize the biology of BCL-2 proteins and the mechanisms of how these proteins are deregulated in distinct B-NHL subtypes. We describe the mechanism of action of BH3-mimetics and the status of their clinical development in B-NHL. Finally, we summarize the mechanisms of sensitivity/resistance to venetoclax.
Keywords: B-cell leukemia/lymphoma-2 (BCL-2); apoptosis; non-Hodgkin lymphomas (NHL); venetoclax.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

