Co-crystals, Salts or Mixtures of Both? The Case of Tenofovir Alafenamide Fumarates
- PMID: 32290280
- PMCID: PMC7238255
- DOI: 10.3390/pharmaceutics12040342
Co-crystals, Salts or Mixtures of Both? The Case of Tenofovir Alafenamide Fumarates
Abstract
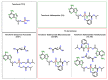
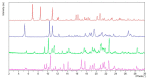
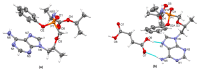
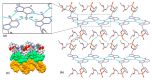
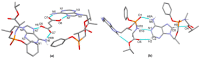
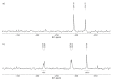
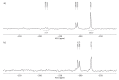
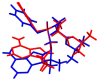
Tenofovir alafenamide fumarate (TAF) is the newest prodrug of tenofovir that constitutes several drug products used for the treatment of HIV/AIDS. Although the solid-state properties of its predecessor tenofovir disoproxil fumarate have been investigated and described in the literature, there are no data in the scientific literature on the solid state properties of TAF. In our report, we describe the preparation of two novel polymorphs II and III of tenofovir alafenamide monofumarate (TA MF2 and TA MF3). The solid-state structure of these compounds was investigated in parallel to the previously known tenofovir alafenamide monofumarate form I (TA MF1) and tenofovir alafenamide hemifumarate (TA HF). Interestingly, the single-crystal X-ray diffraction of TA HF revealed that this derivative exists as a co-crystal form. In addition, we prepared a crystalline tenofovir alafenamide free base (TA) and its hydrochloride salt (TA HCl), which enabled us to determine the structure of TA MF derivatives using 15N-ssNMR (15N-solid state nuclear magnetic resonance). Surprisingly, we observed that TA MF1 exists as a mixed ionization state complex or pure salt, while TA MF2 and TA MF3 can be obtained as pure co-crystal forms.
Keywords: X-ray diffraction; co-crystal; polymorphism; salt; ssNMR; tenofovir alafenamide fumarate.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- The Top 10 Causes of death. [(accessed on 12 March 2020)]; Available online: https://www.who.int/news-room/fact-sheets/detail/the-top-10-causes-of-death.
-
- Global Factsheets 2018 HIV and AIDS Estimates. [(accessed on 12 March 2020)]; Available online: http://aidsinfo.unaids.org/
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

