Do Post-Translational Modifications Influence Protein Aggregation in Neurodegenerative Diseases: A Systematic Review
- PMID: 32290481
- PMCID: PMC7226274
- DOI: 10.3390/brainsci10040232
Do Post-Translational Modifications Influence Protein Aggregation in Neurodegenerative Diseases: A Systematic Review
Abstract
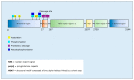
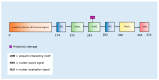
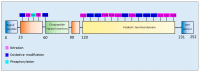
The accumulation of abnormal protein aggregates represents a universal hallmark of neurodegenerative diseases (NDDs). Post-translational modifications (PTMs) regulate protein structure and function. Dysregulated PTMs may influence the propensity for protein aggregation in NDD-proteinopathies. To investigate this, we systematically reviewed the literature to evaluate effects of PTMs on aggregation propensity for major proteins linked to the pathogenesis and/or progression of NDDs. A search of PubMed, MEDLINE, EMBASE, and Web of Science Core Collection was conducted to retrieve studies that investigated an association between PTMs and protein aggregation in seven NDDs: Alzheimer's disease (AD), Parkinson's disease (PD), Huntington's disease (HD), amyotrophic lateral sclerosis (ALS), spinocerebellar ataxias, transmissible spongiform encephalopathy, and multiple sclerosis. Together, 1222 studies were identified, of which 69 met eligibility criteria. We identified that the following PTMs, in isolation or combination, potentially act as modulators of proteinopathy in NDDs: isoaspartate formation in Aβ, phosphorylation of Aβ or tau in AD; acetylation, 4-hydroxy-2-neonal modification, O-GlcNAcylation or phosphorylation of α-synuclein in PD; acetylation or phosphorylation of TAR DNA-binding protein-43 in ALS, and SUMOylation of superoxide dismutase-1 in ALS; and phosphorylation of huntingtin in HD. The potential pharmacological manipulation of these aggregation-modulating PTMs represents an as-yet untapped source of therapy to treat NDDs.
Keywords: neurodegenerative diseases; neurotoxicity; post-translational modifications; protein aggregates.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Wolfe M.S., editor. The Molecular and Cellular Basis of Neurodegenerative Diseases: Underlying Mechanisms. Elsevier; London, UK: 2018. Solving the puzzle of neurodegeneration.
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous

