Ongoing Clinical Trials for the Management of the COVID-19 Pandemic
- PMID: 32291112
- PMCID: PMC7144665
- DOI: 10.1016/j.tips.2020.03.006
Ongoing Clinical Trials for the Management of the COVID-19 Pandemic
Abstract
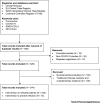
COVID-19 has rapidly developed into a worldwide pandemic with a significant health and economic burden. There are currently no approved treatments or preventative therapeutic strategies. Hundreds of clinical studies have been registered with the intention of discovering effective treatments. Here, we review currently registered interventional clinical trials for the treatment and prevention of COVID-19 to provide an overall summary and insight into the global response.
Keywords: 2019-nCoV; COVID-19; SARS-CoV-2; coronavirus; pandemic.
Copyright © 2020 Elsevier Ltd. All rights reserved.
Figures

Comment in
-
Overwhelming COVID-19 Clinical Trials: Call for Prospective Meta-Analyses.Trends Pharmacol Sci. 2020 Aug;41(8):501-503. doi: 10.1016/j.tips.2020.05.002. Epub 2020 May 20. Trends Pharmacol Sci. 2020. PMID: 32471655 Free PMC article. No abstract available.
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

