Prospective registration and reporting of trial number in randomised clinical trials: global cross sectional study of the adoption of ICMJE and Declaration of Helsinki recommendations
- PMID: 32291261
- PMCID: PMC7190012
- DOI: 10.1136/bmj.m982
Prospective registration and reporting of trial number in randomised clinical trials: global cross sectional study of the adoption of ICMJE and Declaration of Helsinki recommendations
Abstract
Objectives: To evaluate the compliance with prospective registration and inclusion of the trial registration number (TRN) in published randomised controlled trials (RCTs), and to analyse the rationale behind, and detect selective registration bias in, retrospective trial registration.
Design: Cross sectional analysis.
Data sources: PubMed, the 17 World Health Organization's trial registries, University of Toronto library, International Committee of Medical Journal Editors (ICMJE) list of member journals, and the InCites Journal Citation Reports.
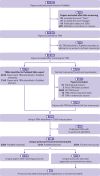
Study selection criteria: RCTs registered in any WHO trial registry and published in any PubMed indexed journal in 2018.
Results: This study included 10 500 manuscripts published in 2105 journals. Overall, 71.2% (7473/10500) reported the TRN and 41.7% (3013/7218) complied with prospective trial registration. The univariable and multivariable analyses reported significant relations (P<0.05) between reporting the TRN and the impact factor and ICMJE membership of the publishing journal. A significant relation (P<0.05) was also observed between prospective trial registration and the registry, region, condition, funding, trial size, interval between paper registration and submission dates, impact factor, and ICMJE membership of the publishing journal. A manuscript published in an ICMJE member journal was 5.8 times more likely to include the TRN (odds ratio 5.8, 95% confidence interval 4.0 to 8.2), and a published trial was 1.8 times more likely to be registered prospectively (1.8, 1.5 to 2.2) when published in an ICMJE member journal compared with other journals. This study detected a new form of bias, selective registration bias, with a higher proportion (85.2% (616/723)) of trials registered retrospectively within a year of submission for publication. Higher rates of retrospective registrations were observed within the first three to eight weeks after enrolment of study participants. Within the 286 RCTs registered retrospectively and published in an ICMJE member journal, only 2.8% (8/286) of the authors included a statement justifying the delayed registration. Reasons included lack of awareness, error of omission, and the registration process taking longer than anticipated.
Conclusions: This study found a high compliance in reporting of the TRN for trial papers published in ICMJE member journals, but prospective trial registration was low.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: All authors have completed the ICMJE uniform disclosure form at www.icmje.org/coi_disclosure.pdf and declare: no support from any organisation for the submitted work in the previous three years; no financial relationships with any organisations that might have an interest in the submitted work; no other relationships or activities that could appear to have influenced the submitted work.
Figures
References
-
- Harrison BA, Mayo-Wilson E. Trial Registration. Res Soc Work Pract 2014;24:372-6. 10.1177/1049731513512374. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources