Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes
- PMID: 32291277
- PMCID: PMC7245348
- DOI: 10.2337/dc19-1892
Effects of Novel Dual GIP and GLP-1 Receptor Agonist Tirzepatide on Biomarkers of Nonalcoholic Steatohepatitis in Patients With Type 2 Diabetes
Abstract
Objective: To determine the effect of tirzepatide, a dual agonist of glucose-dependent insulinotropic polypeptide and glucagon-like peptide 1 receptors, on biomarkers of nonalcoholic steatohepatitis (NASH) and fibrosis in patients with type 2 diabetes mellitus (T2DM).
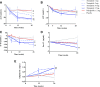
Research design and methods: Patients with T2DM received either once weekly tirzepatide (1, 5, 10, or 15 mg), dulaglutide (1.5 mg), or placebo for 26 weeks. Changes from baseline in alanine aminotransferase (ALT), aspartate aminotransferase (AST), keratin-18 (K-18), procollagen III (Pro-C3), and adiponectin were analyzed in a modified intention-to-treat population.
Results: Significant (P < 0.05) reductions from baseline in ALT (all groups), AST (all groups except tirzepatide 10 mg), K-18 (tirzepatide 5, 10, 15 mg), and Pro-C3 (tirzepatide 15 mg) were observed at 26 weeks. Decreases with tirzepatide were significant compared with placebo for K-18 (10 mg) and Pro-C3 (15 mg) and with dulaglutide for ALT (10, 15 mg). Adiponectin significantly increased from baseline with tirzepatide compared with placebo (10, 15 mg).
Conclusions: In post hoc analyses, higher tirzepatide doses significantly decreased NASH-related biomarkers and increased adiponectin in patients with T2DM.
Trial registration: ClinicalTrials.gov NCT03131687.
© 2020 by the American Diabetes Association.
Conflict of interest statement
This article is featured in a podcast available at https://www.diabetesjournals.org/content/diabetes-core-update-podcasts.
Figures
References
-
- Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease-meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology 2016;64:73–84 - PubMed
-
- Cusi K, Sanyal AJ, Zhang S, et al. Non-alcoholic fatty liver disease (NAFLD) prevalence and its metabolic associations in patients with type 1 diabetes and type 2 diabetes. Diabetes Obes Metab 2017;19:1630–1634 - PubMed
-
- Chalasani N, Younossi Z, Lavine JE, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology 2018;67:328–357 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

