Identification of a Cytopathogenic Toxin from Sneathia amnii
- PMID: 32291280
- PMCID: PMC7283592
- DOI: 10.1128/JB.00162-20
Identification of a Cytopathogenic Toxin from Sneathia amnii
Abstract
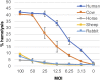
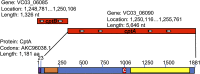
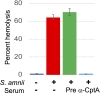
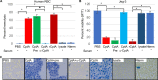
Sneathia amnii is a poorly characterized emerging pathogen that has been implicated in amnionitis and urethritis. We found that S. amnii damages fetal membranes, and we identified and purified a cytotoxic exotoxin that lyses human red blood cells and damages cells from fetal membranes. The gene appears to be cotranscribed with a second gene that encodes a protein with identity to two-partner system transporters, suggesting that it is the "A," or secreted component of a type Vb system. The toxin is 1,881 amino acids with a molecular weight of approximately 200 kDa. It binds to red blood cell membranes and forms pores with a diameter of 2.0 to 3.0 nm, resulting in osmolysis. Because it appears to be the "A" or passenger component of a two-partner system, we propose to name this novel cytotoxin/hemolysin CptA for cytopathogenic toxin component A.IMPORTANCESneathia amnii is a very poorly characterized emerging pathogen that can affect pregnancy outcome and cause urethritis and other infections. To date, nothing is known about its virulence factors or pathogenesis. We have identified and isolated a cytotoxin, named CptA for cytopathogenic toxin, component A, that is produced by S. amnii CptA is capable of permeabilizing chorionic trophoblasts and lysing human red blood cells and, thus, may play a role in virulence. Except for small domains conserved among two-partner secretion system passenger proteins, the cytotoxin exhibits little amino acid sequence homology to known toxins. In this study, we demonstrate the pore-forming activity of this novel toxin.
Keywords: hemolysin; microbiome; pathogenesis; toxins.
Copyright © 2020 American Society for Microbiology.
Figures


References
-
- Fettweis JM, Serrano MG, Brooks JP, Edwards DJ, Girerd PH, Parikh HI, Huang B, Arodz TJ, Edupuganti L, Glascock AL, Xu J, Jimenez NR, Vivadelli SC, Fong SS, Sheth NU, Jean S, Lee V, Bokhari YA, Lara AM, Mistry SD, Duckworth RA, Bradley SP, Koparde VN, Orenda XV, Milton SH, Rozycki SK, Matveyev AV, Wright ML, Huzurbazar SV, Jackson EM, Smirnova E, Korlach J, Tsai Y-C, Dickinson MR, Brooks JL, Drake JI, Chaffin DO, Sexton AL, Gravett MG, Rubens CE, Wijesooriya NR, Hendricks-Muñoz KD, Jefferson KK, Strauss JF, Buck GA. 2019. The vaginal microbiome and preterm birth. Nat Med 25:1012–1021. doi: 10.1038/s41591-019-0450-2. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources

