IDH mutation in glioma: molecular mechanisms and potential therapeutic targets
- PMID: 32291392
- PMCID: PMC7250901
- DOI: 10.1038/s41416-020-0814-x
IDH mutation in glioma: molecular mechanisms and potential therapeutic targets
Abstract
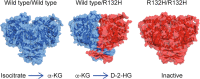
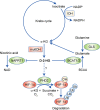
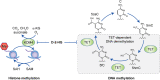
Isocitrate dehydrogenase (IDH) enzymes catalyse the oxidative decarboxylation of isocitrate and therefore play key roles in the Krebs cycle and cellular homoeostasis. Major advances in cancer genetics over the past decade have revealed that the genes encoding IDHs are frequently mutated in a variety of human malignancies, including gliomas, acute myeloid leukaemia, cholangiocarcinoma, chondrosarcoma and thyroid carcinoma. A series of seminal studies further elucidated the biological impact of the IDH mutation and uncovered the potential role of IDH mutants in oncogenesis. Notably, the neomorphic activity of the IDH mutants establishes distinctive patterns in cancer metabolism, epigenetic shift and therapy resistance. Novel molecular targeting approaches have been developed to improve the efficacy of therapeutics against IDH-mutated cancers. Here we provide an overview of the latest findings in IDH-mutated human malignancies, with a focus on glioma, discussing unique biological signatures and proceedings in translational research.
Conflict of interest statement
The authors declare no competing interests.
Figures

References
-
- Koh HJ, Lee SM, Son BG, Lee SH, Ryoo ZY, Chang KT, et al. Cytosolic NADP+-dependent isocitrate dehydrogenase plays a key role in lipid metabolism. J. Biol. Chem. 2004;279:39968–39974. - PubMed
-
- Lee SH, Jo SH, Lee SM, Koh HJ, Song H, Park JW, et al. Role of NADP+-dependent isocitrate dehydrogenase (NADP+-ICDH) on cellular defence against oxidative injury by gamma-rays. Int. J. Radiat. Biol. 2004;80:635–642. - PubMed
-
- Hurley JH, Dean AM, Koshland DE, Jr., Stroud RM. Catalytic mechanism of NADP(+)-dependent isocitrate dehydrogenase: implications from the structures of magnesium-isocitrate and NADP+ complexes. Biochemistry. 1991;30:8671–8678. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

