The landscape of pharmacogenetic testing in a US managed care population
- PMID: 32291400
- PMCID: PMC7332417
- DOI: 10.1038/s41436-020-0788-3
The landscape of pharmacogenetic testing in a US managed care population
Abstract
Purpose: Little is known about how many insured patients receive pharmacogenetic testing. We describe trends of single-gene pharmacogenetic testing in a US managed care population, and demographic and clinical characteristics of patients who received a test.
Methods: We leveraged a random sample of nearly 11 million patients from a data set of paid medical and pharmacy claims to identify patients with at least one claim indicating receipt of at least one of these single-gene pharmacogenetic tests: CYP2C19, CYP2D6, CYP2C9, VKORC1, UGT1A1, and HLA class 1 typing.
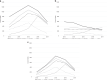
Results: From 1 January 2013 to 30 September 2017, 5712 patients received at least one pharmacogenetic test (55% female; mean age = 43 years). The median number of tests per patient was 3 (mean = 2.7, max = 12); 54% were processed through Managed Medicare/Medicaid, while 45% were processed through commercial insurance. The total number of pharmacogenetic tests received more than doubled from 2013 (n = 1955) to 2015 (n = 4192), then decreased slightly in 2016 (n = 3946). The most common test was CYP2C19 (n = 4719), and "long-term (current) use of other medications" was the most common diagnosis.
Conclusion: Pharmacogenetic testing through patients' insurance was low, but more than doubled from 2013 to 2016. This study highlights the need to better understand utilization patterns and insurance coverage for pharmacogenetic tests.
Keywords: insurance; managed care; pharmacogenetic; pharmacogenomic; testing.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- US National Library of Medicine. Genetics Home Reference. What is precision medicine? https://ghr.nlm.nih.gov/primer/precisionmedicine/definition. Accessed 25 November 2019.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

