Performance of an acoustic settler versus a hollow fiber-based ATF technology for influenza virus production in perfusion
- PMID: 32291490
- PMCID: PMC7228903
- DOI: 10.1007/s00253-020-10596-x
Performance of an acoustic settler versus a hollow fiber-based ATF technology for influenza virus production in perfusion
Abstract
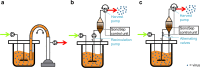
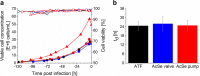
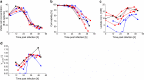
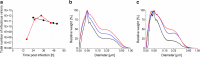
Process intensification and integration is crucial regarding an ever increasing pressure on manufacturing costs and capacities in biologics manufacturing. For virus production in perfusion mode, membrane-based alternating tangential flow filtration (ATF) and acoustic settler are the commonly described cell retention technologies. While acoustic settlers allow for continuous influenza virus harvesting, the use of commercially available membranes for ATF systems typically results in the accumulation of virus particles in the bioreactor vessel. Accordingly, with one single harvest at the end of a cultivation, this increases the risk of lowering the product quality. To assess which cell retention device would be most suitable for influenza A virus production, we compared various key performance figures using AGE1.CR.pIX cells at concentrations between 25 and 50 × 106 cells/mL at similar infection conditions using either an ATF system or an acoustic settler. Production yields, process-related impurities, and aggregation of viruses and other large molecules were evaluated. Taking into account the total number of virions from both the bioreactor and the harvest vessel, a 1.5-3.0-fold higher volumetric virus yield was obtained for the acoustic settler. In addition, fewer large-sized aggregates (virus particles and other molecules) were observed in the harvest taken directly from the bioreactor. In contrast, similar levels of process-related impurities (host cell dsDNA, total protein) were obtained in the harvest for both retention systems. Overall, a clear advantage was observed for continuous virus harvesting after the acoustic settler operation mode was optimized. This development may also allow direct integration of subsequent downstream processing steps. KEY POINTS: • High suspension cell density, immortalized avian cell line, influenza vaccine.
Keywords: Cell culture–based; Influenza; Perfusion; Virus.
Conflict of interest statement
GG, JC, YG, UR declare that they have no conflict of interest. FT has developed the acoustic settler technology. IJ is an employee of ProBioGen AG which has established the AGE1.CR.pIX cell line.
Figures

Similar articles
-
High cell density cultivations by alternating tangential flow (ATF) perfusion for influenza A virus production using suspension cells.Vaccine. 2014 May 19;32(24):2770-81. doi: 10.1016/j.vaccine.2014.02.016. Epub 2014 Feb 25. Vaccine. 2014. PMID: 24583003
-
Influenza A virus production in a single-use orbital shaken bioreactor with ATF or TFF perfusion systems.Vaccine. 2019 Nov 8;37(47):7011-7018. doi: 10.1016/j.vaccine.2019.06.005. Epub 2019 Jun 29. Vaccine. 2019. PMID: 31266669
-
Continuous influenza virus production in a tubular bioreactor system provides stable titers and avoids the "von Magnus effect".PLoS One. 2019 Nov 5;14(11):e0224317. doi: 10.1371/journal.pone.0224317. eCollection 2019. PLoS One. 2019. PMID: 31689309 Free PMC article.
-
Bioreactors for high cell density and continuous multi-stage cultivations: options for process intensification in cell culture-based viral vaccine production.Appl Microbiol Biotechnol. 2016 Mar;100(5):2121-32. doi: 10.1007/s00253-015-7267-9. Epub 2016 Jan 13. Appl Microbiol Biotechnol. 2016. PMID: 26758296 Free PMC article. Review.
-
Bioreactor concepts for cell culture-based viral vaccine production.Expert Rev Vaccines. 2015;14(9):1181-95. doi: 10.1586/14760584.2015.1067144. Epub 2015 Jul 15. Expert Rev Vaccines. 2015. PMID: 26178380 Review.
Cited by
-
Intensified Influenza Virus Production in Suspension HEK293SF Cell Cultures Operated in Fed-Batch or Perfusion with Continuous Harvest.Vaccines (Basel). 2023 Dec 5;11(12):1819. doi: 10.3390/vaccines11121819. Vaccines (Basel). 2023. PMID: 38140223 Free PMC article.
-
Digital Twin for Continuous Production of Virus-like Particles toward Autonomous Operation.ACS Omega. 2024 Jul 25;9(32):34990-35013. doi: 10.1021/acsomega.4c04985. eCollection 2024 Aug 13. ACS Omega. 2024. PMID: 39157157 Free PMC article.
-
Enhancing and stabilizing monoclonal antibody production by Chinese hamster ovary (CHO) cells with optimized perfusion culture strategies.Front Bioeng Biotechnol. 2023 Jan 20;11:1112349. doi: 10.3389/fbioe.2023.1112349. eCollection 2023. Front Bioeng Biotechnol. 2023. PMID: 36741761 Free PMC article.
-
Navigating the Purification Process: Maintaining the Integrity of Replication-Competent Enveloped Viruses.Vaccines (Basel). 2025 Apr 23;13(5):444. doi: 10.3390/vaccines13050444. Vaccines (Basel). 2025. PMID: 40432057 Free PMC article. Review.
-
Production of Modified Vaccinia Ankara Virus by Intensified Cell Cultures: A Comparison of Platform Technologies for Viral Vector Production.Biotechnol J. 2021 Jan;16(1):e2000024. doi: 10.1002/biot.202000024. Epub 2020 Sep 8. Biotechnol J. 2021. PMID: 32762152 Free PMC article.
References
-
- Agarabi CD, Chavez BK, Lute SC, Read EK, Rogstad S, Awotwe-Otoo D, Brown MR, Boyne MT, 2nd, Brorson KA. Exploring the linkage between cell culture process parameters and downstream processing utilizing a Plackett-Burman design for a model monoclonal antibody. Biotechnol Prog. 2017;33(1):163–170. doi: 10.1002/btpr.2402. - DOI - PubMed
-
- Blaschczok K, Kaiser SC, Löffelholz C, Imseng N, Burkart J, Bösch P, Dornfeld W, Eibl R, Eibl D. Investigations on mechanical stress caused to CHO suspension cells by standard and single-use pumps. Chem Ing Tech. 2013;85(1–2):144–152. doi: 10.1002/cite.201200135. - DOI
-
- Chen C, Wong HE, Goudar CT. Upstream process intensification and continuous manufacturing. Curr Opin Chem Eng. 2018;22:191–198. doi: 10.1016/j.coche.2018.10.006. - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

