Placental endovascular extravillous trophoblasts (enEVTs) educate maternal T-cell differentiation along the maternal-placental circulation
- PMID: 32291850
- PMCID: PMC7260064
- DOI: 10.1111/cpr.12802
Placental endovascular extravillous trophoblasts (enEVTs) educate maternal T-cell differentiation along the maternal-placental circulation
Abstract
Objectives: During human pregnancy, the endothelial cells of the uterine spiral arteries (SPA) are extensively replaced by a subtype of placental trophoblasts, endovascular extravillous trophoblasts (enEVTs), thus establishing a placental-maternal circulation. On this pathway, foetus-derived placental villi and enEVTs bath into the maternal blood that perfuses along SPA being not attacked by the maternal lymphocytes. We aimed to reveal the underlying mechanism of such immune tolerance.
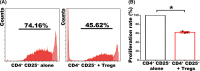
Methods: In situ hybridization, immunofluorescence, ELISA and FCM assay were performed to examine TGF-β1 expression and distribution of regulatory T cells (Tregs) along the placental-maternal circulation route. The primary enEVTs, interstitial extravillous trophoblasts (iEVTs) and decidual endothelial cells (dECs) were purified by FACS, and their conditioned media were collected to treat naïve CD4+ T cells. Treg differentiation was measured by FLOW and CFSE assays.
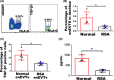
Results: We found that enEVTs but not iEVTs or dECs actively produced TGF-β1. The primary enEVTs significantly promoted naïve CD4+ T-cell differentiation into immunosuppressive FOXP3+ Tregs, and this effect was dependent on TGF-β1. In recurrent spontaneous abortion (RSA) patients, an evidently reduced proportion of TGF-β1-producing enEVTs and their ability to educate Tregs differentiation were observed.
Conclusions: Our findings demonstrate a unique immune-regulatory characteristic of placental enEVTs to develop immune tolerance along the placental-maternal circulation. New insights into the pathogenesis of RSA are also suggested.
Keywords: RSA; TGF-β1; Tregs; enEVTs; placental-maternal circulation.
© 2020 The Authors. Cell Proliferation Published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no competing interests.
Figures




References
-
- De Carolis C, Perricone C, Perricone R. War and peace at the feto‐placental front line: recurrent spontaneous abortion. Isr Med Assoc J. 2014;16:667‐668. - PubMed
-
- Sargent IL, Wilkins T, Redman CW. Maternal immune responses to the fetus in early pregnancy and recurrent miscarriage. Lancet. 1988;2:1099‐1104. - PubMed
-
- Krieg S, Westphal L. Immune function and recurrent pregnancy loss. Semin Reprod Med. 2015;33:305‐312. - PubMed
-
- Clifford K, Rai R, Watson H, Regan L. An informative protocol for the investigation of recurrent miscarriage: preliminary experience of 500 consecutive cases. Hum Reprod. 1994;9:1328‐1332. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

