Characterization of Esterase Genes Involving Malathion Detoxification and Establishment of an RNA Interference Method in Liposcelis bostrychophila
- PMID: 32292357
- PMCID: PMC7118802
- DOI: 10.3389/fphys.2020.00274
Characterization of Esterase Genes Involving Malathion Detoxification and Establishment of an RNA Interference Method in Liposcelis bostrychophila
Abstract
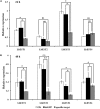
Esterases (ESTs) play important roles in metabolizing various physiologically endogenous and exogenous compounds, and various environmental xenobiotics in insects. The psocid, Liposcelis bostrychophila is a major pest of stored products worldwide and rapidly develops resistance to commonly insecticides. However, the involvement of ESTs in insecticide metabolization and the application of RNAi approach in psocids have not been well elucidated. In this study, we characterized four LbEST genes and investigated the transcriptional levels of these genes at different developmental stages and under different insecticides exposures to assess their potential roles in response to insecticides. The four LbESTs contain a catalytic triad (Ser-His-Glu) linked to an oxyanion hole and acyl pocket involved in substrate stabilization during its hydrolysis. Synergism observed with the esterase-inhibitor DEF suggests the involvement of esterases in malathion detoxification. LbESTs were expressed during the whole of developmental stages, but predominant abundance in the first nymphal instar and adult stage. The mRNA level of three LbEST genes (except for LbEST4) was induced (1.29- to 5.60 fold) in response to malathion or deltamethrin exposures, indicating that these esterases are involved in the detoxification process. Silencing of LbEST1, LbEST2 or LbEST3 through dsRNA feeding led to a higher mortality of psocids upon the malathion treatment compared to controls (1.83 to 2.69-fold), demonstrating that these esterase genes play roles in malathion detoxification in L. bostrychophila. Our study provides new evidence for understanding of the function and regulation mechanism of esterases in L. bostrychophila in insecticide detoxification. The current study also suggests that the present RNAi method could be applied for gene functional studies in psocids.
Keywords: RNAi; booklice; detoxification enzyme; microsatellite; stored product pests.
Copyright © 2020 Wei, He, Miao, Tu, Wang, Dou and Wang.
Figures





References
-
- Asperen K. V. (1962). A study of housefly esterases by means of a sensitive colorimetric method. J. Insect Physiol. 8 401–414. 10.1016/0022-1910(62)90074-4 - DOI
LinkOut - more resources
Full Text Sources

