Tumor circulome in the liquid biopsies for cancer diagnosis and prognosis
- PMID: 32292514
- PMCID: PMC7150480
- DOI: 10.7150/thno.40532
Tumor circulome in the liquid biopsies for cancer diagnosis and prognosis
Abstract
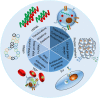
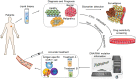
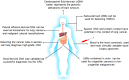
Liquid biopsy is a convenient, fast, non-invasive and reproducible sampling method that can dynamically reflect the changes in tumor gene expression profile, and provide a robust basis for individualized therapy and early diagnosis of cancer. Circulating tumor DNA (ctDNA) and circulating tumor cells (CTCs) are the currently approved diagnostic biomarkers for screening cancer patients. In addition, tumor-derived extracellular vesicles (tdEVs), circulating tumor-derived proteins, circulating tumor RNA (ctRNA) and tumor-bearing platelets (TEPs) are other components of liquid biopsies with diagnostic potential. In this review, we have discussed the clinical applications of these biomarkers, and the factors that limit their implementation in routine clinical practice. In addition, the most recent developments in the isolation and analysis of circulating tumor biomarkers have been summarized, and the potential of non-blood liquid biopsies in tumor diagnostics has also been discussed.
Keywords: liquid biopsy; tumor circulome; tumor screening.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures

References
-
- De Rubis G, Rajeev Krishnan S, Bebawy M. Liquid biopsies in cancer diagnosis, monitoring, and prognosis. Trends Pharmacol Sci. 2019;40:172–186. - PubMed
-
- Lopez A, Harada K, Mizrak Kaya D, Dong X, Song S, Ajani JA. Liquid biopsies in gastrointestinal malignancies: when is the big day? Expert Rev Anticancer Ther. 2018;18:19–38. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

