Tumor reoxygenation for enhanced combination of radiation therapy and microwave thermal therapy using oxygen generation in situ by CuO nanosuperparticles under microwave irradiation
- PMID: 32292521
- PMCID: PMC7150478
- DOI: 10.7150/thno.42818
Tumor reoxygenation for enhanced combination of radiation therapy and microwave thermal therapy using oxygen generation in situ by CuO nanosuperparticles under microwave irradiation
Abstract
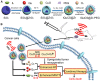
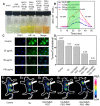
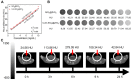
As known, radiation therapy (RT) can exacerbate the degree of hypoxia of tumor cells, which induces serious resistance to RT and in turn, is the greatest obstacle to RT. Reoxygenation can restore the hypoxic state of tumor cells, which plays an important role in reshaping tumor microenviroment for achieving optimal therapeutic efficacy. Herein, we report for the first time that microwave (MW)-triggered IL-Quercetin-CuO-SiO2@ZrO2-PEG nanosuperparticles (IQuCS@Zr-PEG NSPs) have been used to achieve an optimal RT therapeutic outcomes by the strategy of upregulating tumor reoxygenation, i.e. hypoxic cells acquire oxygen and return to normal state. Methods: We prepared a promising multifunctional nanosuperparticle to upregulate tumor reoxygenation by utilizing CuO nanoparticle to generate oxygen under MW irradiation in the tumor microenvironment. The IQuCS@Zr-PEG NSPs were obtained by introducing CuO nanoparticles, MW sensitizer of 1-butyl-3-methylimidazolium hexafluorophosphate (IL), radiosensitizer of Quercetin (Qu) and surface modifier of monomethoxy polyethylene glycol sulfhyl (mPEG-SH, 5k Da) into mesoporous sandwich SiO2@ZrO2 nanosuperparticles (SiO2@ZrO2 NSPs). The release oxygen by IQuCS@Zr-PEG NSPs under MW irradiation was investigated by a microcomputer dissolved oxygen-biochemical oxygen demand detector (DO-BOD) test. Finally, we used the 99mTc-HL91 labeled reoxygenation imaging, Cellular immunofluorescence, immunohistochemistry, and TUNEL experiments to verify that this unique MW-responsive reoxygenation enhancer can be used to stimulate reshaping of the tumor microenvironment. Results: Through experiments we found that the IQuCS@Zr-PEG NSPs can persistently release oxygen under the MW irradiation, which upregulates tumor reoxygenation and improve the combined tumor treatment effect of RT and microwave thermal therapy (MWTT). Cellular immunofluorescence and immunohistochemistry experiments demonstrated that the IQuCS@Zr-PEG NSPs can downregulate the expression of hypoxia-inducible factor 1α (HIF-1α) under MW irradiation. The 99mTc-HL91 labeled reoxygenation imaging experiment also showed that the oxygen generated by IQuCS@Zr-PEG NSPs under MW irradiation can significantly increase the reoxygenation capacity of tumor cells, thus reshaping the tumor microenvironment. The high inhibition rate of 98.62% was achieved in the antitumor experiments in vivo. In addition, the IQuCS@Zr-PEG NSPs also had good computed tomography (CT) imaging effects, which can be used to monitor the treatment of tumors in real-time. Conclusions: The proof-of-concept strategy of upregulating tumor reoxygenation is achieved by MW triggered IQuCS@Zr-PEG NSPs, which has exhibited optimal therapeutic outcomes of combination of RT and MWTT tumor. Such unique MW-responsive reoxygenation enhancer may stimulate the research of reshaping tumor microenvironment for enhancing versatile tumor treatment.
Keywords: microwave; radiation therapy; reoxygenation; thermal therapy; tumor.
© The author(s).
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures




Similar articles
-
Oxygen Production of Modified Core-Shell CuO@ZrO2 Nanocomposites by Microwave Radiation to Alleviate Cancer Hypoxia for Enhanced Chemo-Microwave Thermal Therapy.ACS Nano. 2018 Dec 26;12(12):12721-12732. doi: 10.1021/acsnano.8b07749. Epub 2018 Dec 6. ACS Nano. 2018. PMID: 30512923
-
Mitochondria-targeted zirconium metal-organic frameworks for enhancing the efficacy of microwave thermal therapy against tumors.Biomater Sci. 2018 May 29;6(6):1535-1545. doi: 10.1039/c8bm00142a. Biomater Sci. 2018. PMID: 29670952
-
Dual-Functional Supernanoparticles with Microwave Dynamic Therapy and Microwave Thermal Therapy.Nano Lett. 2019 Aug 14;19(8):5277-5286. doi: 10.1021/acs.nanolett.9b01735. Epub 2019 Jul 24. Nano Lett. 2019. PMID: 31331173
-
Micro-Nanomaterials for Tumor Microwave Hyperthermia: Design, Preparation, and Application.Curr Drug Deliv. 2017;14(3):307-322. doi: 10.2174/1567201813666160108113805. Curr Drug Deliv. 2017. PMID: 26743355 Review.
-
Hyperthermia can alter tumor physiology and improve chemo- and radio-therapy efficacy.Adv Drug Deliv Rev. 2020;163-164:98-124. doi: 10.1016/j.addr.2020.07.007. Epub 2020 Jul 15. Adv Drug Deliv Rev. 2020. PMID: 32681862
Cited by
-
Endogenous H2O2 Self-Replenishment and Sustainable Cascades Enhance the Efficacy of Sonodynamic Therapy.Int J Nanomedicine. 2023 Nov 13;18:6667-6687. doi: 10.2147/IJN.S431221. eCollection 2023. Int J Nanomedicine. 2023. PMID: 38026520 Free PMC article.
-
Hemoglobin-loaded hollow mesoporous carbon-gold nanocomposites enhance microwave ablation through hypoxia relief.J Nanobiotechnology. 2025 Apr 30;23(1):326. doi: 10.1186/s12951-025-03387-x. J Nanobiotechnology. 2025. PMID: 40307855 Free PMC article.
-
Microwaves, a potential treatment for bacteria: A review.Front Microbiol. 2022 Jul 25;13:888266. doi: 10.3389/fmicb.2022.888266. eCollection 2022. Front Microbiol. 2022. PMID: 35958124 Free PMC article. Review.
-
Bioengineering of CuO porous (nano)particles: role of surface amination in biological, antibacterial, and photocatalytic activity.Sci Rep. 2022 Sep 12;12(1):15351. doi: 10.1038/s41598-022-19553-2. Sci Rep. 2022. PMID: 36097028 Free PMC article.
-
Nano-engineering nanomedicines with customized functions for tumor treatment applications.J Nanobiotechnology. 2023 Aug 2;21(1):250. doi: 10.1186/s12951-023-01975-3. J Nanobiotechnology. 2023. PMID: 37533106 Free PMC article. Review.
References
-
- Veire SVD, Stalmans I, Heindryckx F, Oura H, Tijeras-Raballand A, Schmidt T. et al. Further pharmacological and genetic evidence for the efficacy of PlGF inhibition in cancer and eye disease. Cell. 2010;141:178–190. - PubMed
-
- Zhou ZY, Song X, Jia R, Wang H, Dai L, Xu X. et al. Radiation-inducible human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) gene therapy: a novel treatment for radioresistant uveal melanoma. Pigment Cell Melanoma Res. 2010;23:661–674. - PubMed
-
- Eisbruch A. Intensity-modulated radiation therapy in the treatment of head and neck cancer. Nat Clin Pract Oncol. 2005;2:34–39. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

