Synthesis and Evaluation of Noncovalent Naphthalene-Based KEAP1-NRF2 Inhibitors
- PMID: 32292559
- PMCID: PMC7153276
- DOI: 10.1021/acsmedchemlett.9b00631
Synthesis and Evaluation of Noncovalent Naphthalene-Based KEAP1-NRF2 Inhibitors
Abstract
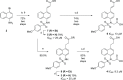
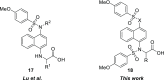
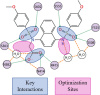
The oxidative stress response, gated by the protein-protein interaction of KEAP1 and NRF2, has garnered significant interest in the past decade. Misregulation in this pathway has been implicated in disease states such as multiple sclerosis, rheumatoid arthritis, and diabetic chronic wounds. Many of the known activators of NRF2 are electrophilic in nature and may operate through several biological pathways rather than solely through the activation of the oxidative stress response. Recently, our lab has reported a nonelectrophilic, monoacidic, naphthalene-based NRF2 activator which exhibited good potency in vitro. Herein, we report a detailed structure-activity relationship of naphthalene-based NRF2 activators, an X-ray crystal structure of our monoacidic KEAP1 inhibitor, and identification of an underexplored area of the NRF2 binding pocket of KEAP1.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures







References
-
- Rabbani P. S.; Ellison T.; Waqas B.; Sultan D.; Abdou S.; David J. A.; Cohen J. M.; Gomez-Viso A.; Lam G.; Kim C.; Thomson J.; Ceradini D. J. Targeted Nrf2 activation therapy with RTA 408 enhances regenerative capacity of diabetic wounds. Diabetes Res. Clin. Pract. 2018, 139, 11–23. 10.1016/j.diabres.2018.02.021. - DOI - PubMed
-
- Gopal S.; Mikulskis A.; Gold R.; Fox R. J.; Dawson K. T.; Amaravadi L. Evidence of activation of the Nrf2 pathway in multiple sclerosis patients treated with delayed-release dimethyl fumarate in the phase 3 DEFINE and CONFIRM studies. Multiple Sclerosis Journal 2017, 23, 1875–1883. 10.1177/1352458517690617. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Chemical Information
Miscellaneous

