Expanding the Medicinal Chemist Toolbox: Comparing Seven C(sp2)-C(sp3) Cross-Coupling Methods by Library Synthesis
- PMID: 32292569
- PMCID: PMC7153271
- DOI: 10.1021/acsmedchemlett.0c00093
Expanding the Medicinal Chemist Toolbox: Comparing Seven C(sp2)-C(sp3) Cross-Coupling Methods by Library Synthesis
Abstract
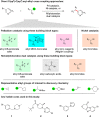
Despite recent advances in the field of C(sp2)-C(sp3) cross-couplings and the accompanying increase in publications, it can be hard to determine which method is appropriate for a given reaction when using the highly functionalized intermediates prevalent in medicinal chemistry. Thus a study was done comparing the ability of seven methods to directly install a diverse set of alkyl groups on "drug-like" aryl structures via parallel library synthesis. Each method showed substrates that it excelled at coupling compared with the other methods. When analyzing the reactions run across all of the methods, a reaction success rate of 50% was achieved. Whereas this is promising, there are still gaps in the scope of direct C(sp2)-C(sp3) coupling methods, like tertiary group installation. The results reported herein should be used to inform future syntheses, assess reaction scope, and encourage medicinal chemists to expand their synthetic toolbox.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



References
LinkOut - more resources
Full Text Sources
Other Literature Sources

