First-in-human study of anticancer immunotherapy drug candidate mobilan: safety, pharmacokinetics and pharmacodynamics in prostate cancer patients
- PMID: 32292576
- PMCID: PMC7147088
- DOI: 10.18632/oncotarget.27549
First-in-human study of anticancer immunotherapy drug candidate mobilan: safety, pharmacokinetics and pharmacodynamics in prostate cancer patients
Abstract
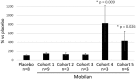
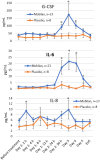
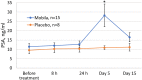
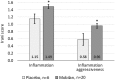
Toll-like receptor 5 (TLR5) controls endogenous immune responses to pathogens and is a promising target for pharmacological stimulation of anti-tumor immunity. Mobilan is an innovative gene therapy agent consisting of a non-replicating bicistronic adenovirus directing constitutive expression of human Toll-like receptor 5 (TLR5) and the secreted flagellin-based TLR5 agonist, 502s. In mice, Mobilan injection into prostate tumors resulted in autocrine TLR5 signaling, immune system activation, and suppression of tumor growth and metastasis. Here we report a first-in-human placebo-controlled clinical study of Mobilan aimed at evaluating safety, tolerability, pharmacokinetics and pharmacodynamics of a single intra-prostate injection of Mobilan in early stage prostate cancer patients. Mobilan was safe and well-tolerated at all tested doses; thus, the maximum tolerated dose was not identified. Injection of Mobilan induced signs of self-resolving inflammation not present in placebo-injected patients, including transient elevation of PSA and cytokine (G-CSF, IL-6) levels, and increased lymphoid infiltration in prostate tissue. The highest dose of Mobilan (1011 viral particles) produced the best combination of safety and pharmacodynamic effects. Therefore, Mobilan is well-tolerated and induces the expected pharmacodynamic response in humans. These results support further clinical development of Mobilan as a novel immunotherapy for prostate cancer.
Keywords: adenoviral vector; immunotherapy; intratumor injection; mobilan; prostate cancer.
Conflict of interest statement
CONFLICTS OF INTEREST There are no conflicts of interest related to this study.
Figures


References
-
- Heidenreich A, Bellmunt J, Bolla M, Joniau S, Mason M, Matveev V, Mottet N, Schmid HP, van der Kwast T, Wiegel T, Zattoni F; European Association of Urology. EAU guidelines on prostate cancer. Part 1: screening, diagnosis, and treatment of clinically localised disease. European Urology. 2011; 59:61–71. 10.1016/j.eururo.2010.10.039. - DOI - PubMed
-
- Gillessen S, Attard G, Beer TM, Beltran H, Bossi A, Bristow R, Carver B, Castellano D, Chung BH, Clarke N, Daugaard G, Davis ID, de Bono J, et al.. Management of Patients with Advanced Prostate Cancer: The Report of the Advanced Prostate Cancer Consensus Conference APCCC 2017. Eur Urol. 2018; 73:178–211. 10.1016/j.eururo.2017.06.002. - DOI - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

