Recent advances and perspectives of nucleic acid detection for coronavirus
- PMID: 32292623
- PMCID: PMC7102540
- DOI: 10.1016/j.jpha.2020.02.010
Recent advances and perspectives of nucleic acid detection for coronavirus
Abstract
The recent pneumonia outbreak caused by a novel coronavirus (SARS-CoV-2) is posing a great threat to global public health. Therefore, rapid and accurate identification of pathogenic viruses plays a vital role in selecting appropriate treatments, saving people's lives and preventing epidemics. It is important to establish a quick standard diagnostic test for the detection of the infectious disease (COVID-19) to prevent subsequent secondary spread. Polymerase chain reaction (PCR) is regarded as a gold standard test for the molecular diagnosis of viral and bacterial infections with high sensitivity and specificity. Isothermal nucleic acid amplification is considered to be a highly promising candidate method due to its fundamental advantage in quick procedure time at constant temperature without thermocycler operation. A variety of improved or new approaches also have been developed. This review summarizes the currently available detection methods for coronavirus nucleic acid. It is anticipated that this will assist researchers and clinicians in developing better techniques for timely and effective detection of coronavirus infection.
Keywords: Coronavirus; Isothermal nucleic acid amplification-based methods; Microarray-based methods; Nucleic acid detection; PCR-Based methods.
© 2020 Xi'an Jiaotong University. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare that there are no conflicts of interest.
Figures

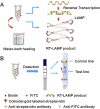

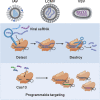
References
-
- Kim H., Park M., Hwang J. Development of label-free colorimetric assay for MERS-CoV using gold nanoparticles. ACS Sens. 2019;4:1306–1312. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

