Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment
- PMID: 32292627
- PMCID: PMC7102606
- DOI: 10.1016/j.eng.2020.02.006
Clinical Study of Mesenchymal Stem Cell Treatment for Acute Respiratory Distress Syndrome Induced by Epidemic Influenza A (H7N9) Infection: A Hint for COVID-19 Treatment
Abstract
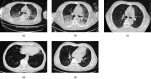
H7N9 viruses quickly spread between mammalian hosts and carry the risk of human-to-human transmission, as shown by the 2013 outbreak. Acute respiratory distress syndrome (ARDS), lung failure, and acute pneumonia are major lung diseases in H7N9 patients. Transplantation of mesenchymal stem cells (MSCs) is a promising choice for treating virus-induced pneumonia, and was used to treat H7N9-induced ARDS in 2013. The transplant of MSCs into patients with H7N9-induced ARDS was conducted at a single center through an open-label clinical trial. Based on the principles of voluntariness and informed consent, 44 patients with H7N9-induced ARDS were included as a control group, while 17 patients with H7N9-induced ARDS acted as an experimental group with allogeneic menstrual-blood-derived MSCs. It was notable that MSC transplantation significantly lowered the mortality of the experimental group, compared with the control group (17.6% died in the experimental group while 54.5% died in the control group). Furthermore, MSC transplantation did not result in harmful effects in the bodies of four of the patients who were part of the five-year follow-up period. Collectively, these results suggest that MSCs significantly improve the survival rate of H7N9-induced ARDS and provide a theoretical basis for the treatment of H7N9-induced ARDS in both preclinical research and clinical studies. Because H7N9 and the coronavirus disease 2019 (COVID-19) share similar complications (e.g., ARDS and lung failure) and corresponding multi-organ dysfunction, MSC-based therapy could be a possible alternative for treating COVID-19.
Keywords: Acute respiratory distress syndrome; COVID-19; Epidemic Influenza A; H7N9; Mesenchymal stem cell; Stem cell therapeutics.
© 2020 THE AUTHORS.
Figures
References
-
- Swayne D.E., Suarez D.L. Highly pathogenic avian influenza. Rev Sci Technol. 2000;19:463–482. - PubMed
-
- Gao R., Cao B., Hu Y., Feng Z., Wang D., Hu W. Human infection with a novel avian-origin influenza A (H7N9) virus. N Engl J Med. 2013;368:1888–1897. - PubMed
-
- Liu D., Shi W., Shi Y., Wang D., Xiao H., Li W. Origin and diversity of novel avian influenza A H7N9 viruses causing human infection: phylogenetic, structural, and coalescent analyses. Lancet. 2013;381:1926–1932. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
