Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19
- PMID: 32293003
- PMCID: PMC7184480
- DOI: 10.1093/cvr/cvaa097
Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19
Erratum in
-
Erratum to: Hypertension, the renin-angiotensin system, and the risk of lower respiratory tract infections and lung injury: implications for COVID-19: European Society of Hypertension COVID-19 Task Force Review of Evidence.Cardiovasc Res. 2021 Sep 28;117(11):2394. doi: 10.1093/cvr/cvab224. Cardiovasc Res. 2021. PMID: 34269396 Free PMC article. No abstract available.
Abstract
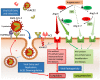
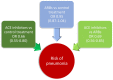
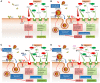
Systemic arterial hypertension (referred to as hypertension herein) is a major risk factor of mortality worldwide, and its importance is further emphasized in the context of the novel severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection referred to as COVID-19. Patients with severe COVID-19 infections commonly are older and have a history of hypertension. Almost 75% of patients who have died in the pandemic in Italy had hypertension. This raised multiple questions regarding a more severe course of COVID-19 in relation to hypertension itself as well as its treatment with renin-angiotensin system (RAS) blockers, e.g. angiotensin-converting enzyme inhibitors (ACEIs) and angiotensin receptor blockers (ARBs). We provide a critical review on the relationship of hypertension, RAS, and risk of lung injury. We demonstrate lack of sound evidence that hypertension per se is an independent risk factor for COVID-19. Interestingly, ACEIs and ARBs may be associated with lower incidence and/or improved outcome in patients with lower respiratory tract infections. We also review in detail the molecular mechanisms linking the RAS to lung damage and the potential clinical impact of treatment with RAS blockers in patients with COVID-19 and a high cardiovascular and renal risk. This is related to the role of angiotensin-converting enzyme 2 (ACE2) for SARS-CoV-2 entry into cells, and expression of ACE2 in the lung, cardiovascular system, kidney, and other tissues. In summary, a critical review of available evidence does not support a deleterious effect of RAS blockers in COVID-19 infections. Therefore, there is currently no reason to discontinue RAS blockers in stable patients facing the COVID-19 pandemic.
Keywords: Angiotensin; COVID-19; Cardiovascular; Hypertension; Lung.
Published on behalf of the European Society of Cardiology. All rights reserved. © The Author(s) 2020. For permissions, please email: journals.permissions@oup.com.
Figures

References
-
- Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, Xiang J, Wang Y, Song B, Gu X, Guan L, Wei Y, Li H, Wu X, Xu J, Tu S, Zhang Y, Chen H, Cao B.. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020;395:1054–1062. - PMC - PubMed
-
- Zhou P, Yang XL, Wang XG, Hu B, Zhang L, Zhang W, Si HR, Zhu Y, Li B, Huang CL, Chen HD, Chen J, Luo Y, Guo H, Jiang RD, Liu MQ, Chen Y, Shen XR, Wang X, Zheng XS, Zhao K, Chen QJ, Deng F, Liu LL, Yan B, Zhan FX, Wang YY, Xiao GF, Shi ZL.. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020;579:270–273. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

