Trends in prevalence of comorbidities in heart failure clinical trials
- PMID: 32293090
- PMCID: PMC7906002
- DOI: 10.1002/ejhf.1818
Trends in prevalence of comorbidities in heart failure clinical trials
Abstract
Aims: The primary objective of this systematic review was to estimate the prevalence and temporal changes in chronic comorbid conditions reported in heart failure (HF) clinical trials.
Methods and results: We searched MEDLINE for HF trials enrolling more than 400 patients published between 2001 and 2016.Trials were divided into HF with reduced ejection fraction (HFrEF), HF with preserved ejection fraction (HFpEF), or trials enrolling regardless of ejection fraction. The prevalence of baseline chronic comorbid conditions was categorized according to the algorithm proposed by the Chronic Conditions Data Warehouse, which is used to analyse Medicare data. To test for a trend in the prevalence of comorbid conditions, linear regression models were used to evaluate temporal trends in prevalence of comorbidities. Overall, 118 clinical trials enrolling a cumulative total of 215 508 patients were included. Across all comorbidities examined, data were reported in a mean of 35% of trials, without significant improvement during the study period. Reporting of comorbidities was more common in HFrEF trials (51%) compared with HFpEF trials (27%). Among trials reporting data, hypertension (63%), ischaemic heart disease (44%), hyperlipidaemia (48%), diabetes (33%), chronic kidney disease (25%) and atrial fibrillation (25%) were the major comorbidities. The prevalence of comorbidities including hypertension, atrial fibrillation and chronic kidney disease increased over time while the prevalence of smoking decreased in HFrEF trials.
Conclusion: Many HF trials do not report baseline comorbidities. A more rigorous, systematic, and standardized framework needs to be adopted for future clinical trials to ensure adequate comorbidity reporting and improve recruitment of multi-morbid HF patients.
Keywords: Clinical trials; Comorbidities; Heart failure; Trends.
© 2020 European Society of Cardiology.
Figures
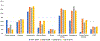


Comment in
-
The unbearable underreporting of comorbidities in heart failure clinical trials.Eur J Heart Fail. 2020 Jun;22(6):1043-1044. doi: 10.1002/ejhf.1846. Epub 2020 Apr 29. Eur J Heart Fail. 2020. PMID: 32351008 No abstract available.
References
-
- Iyngkaran P, Majoni W, Cass A, Sanders P, Ronco C, Brady S, Kangaharan N, Ilton M, Hare DL, Thomas MC. Northern territory perspectives on heart failure with comorbidities – understanding trial validity and exploring collaborative opportunities to broaden the evidence base. Heart Lung Circ 2015;24:536–543. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

