Abnormal mitosis in reactive astrocytes
- PMID: 32293551
- PMCID: PMC7158149
- DOI: 10.1186/s40478-020-00919-4
Abnormal mitosis in reactive astrocytes
Abstract
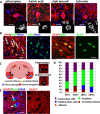
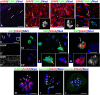
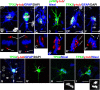
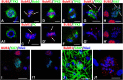
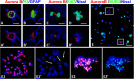
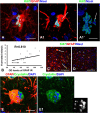
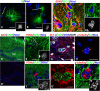
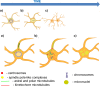
Although abnormal mitosis with disarranged metaphase chromosomes or many micronuclei in astrocytes (named "Alzheimer I type astrocytes" and later "Creutzfeldt-Peters cells") have been known for nearly 100 years, the origin and mechanisms of this pathology remain elusive. In experimental brain insults in rats, we show that abnormal mitoses that are not followed by cytokinesis are typical for reactive astrocytes. The pathology originates due to the inability of the cells to form normal mitotic spindles with subsequent metaphase chromosome congression, which, in turn may be due to shape constraints aggravated by cellular enlargement and to the accumulation of large amounts of cytosolic proteins. Many astrocytes escape from arrested mitosis by producing micronuclei. These polyploid astrocytes can survive for long periods of time and enter into new cell cycles.
Keywords: Astrocytes; Creutzfeldt-Peters cells; Mitosis; Mitotic spindles; Polyploidy.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures




References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

