Use of Temporary Mechanical Circulatory Support for Management of Cardiogenic Shock Before and After the United Network for Organ Sharing Donor Heart Allocation System Changes
- PMID: 32293644
- PMCID: PMC7160750
- DOI: 10.1001/jamacardio.2020.0692
Use of Temporary Mechanical Circulatory Support for Management of Cardiogenic Shock Before and After the United Network for Organ Sharing Donor Heart Allocation System Changes
Abstract
Importance: The new United Network for Organ Sharing (UNOS) donor heart allocation system gives priority to patients supported with nondischargeable mechanical circulatory support (MCS) devices while awaiting heart transplant. Whether there has been a change in temporary MCS use in cardiac intensive care units (CICUs) since the implementation of this policy is unknown.
Objectives: To examine whether the UNOS donor heart allocation system revision in October 2018 was associated with changes in temporary MCS use in CICUs and whether temporary MCS use differed between US transplant centers and US nontransplant centers and Canadian centers.
Design, setting, and participants: In this cohort study, 14 centers from the Critical Care Cardiology Trials Network (CCCTN), a multicenter network of tertiary CICUs in North America, contributed 2-month snapshots of consecutive medical CICU admissions between September 1, 2017, and September 1, 2018 (prerevision period), and October 1, 2018, and September 1, 2019 (postrevision period). CICUs were classified as US transplant centers (n = 7) or other CICUs (US nontransplant centers or Canadian centers; n = 7).
Exposure: Revision to the UNOS donor heart allocation system.
Main outcomes and measures: Treatment with temporary MCS (intra-aortic balloon pump, microaxial intracardiac ventricular assist device, percutaneous centrifugal ventricular assist device, venoarterial extracorporeal membrane oxygenation, or surgically implanted, nondischargeable MCS device) during hospital admission.
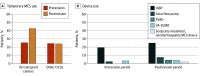
Results: A total of 384 admissions for acute, decompensated, heart failure-related cardiogenic shock (ADHF-CS) were included, among which 248 (64.6%) were to US transplant centers; 126 admissions (51%) were in the prerevision period and 122 (49%) were in the postrevision period. The mean (SD) patient age was 61.2 (14.6) years; 246 patients (64.1%) were male. The proportion of admissions with ADHF-CS managed with temporary MCS at US transplant centers significantly increased from 25.4% (32 of 126 admissions) before to 42.6% (52 of 122 admissions) after the UNOS allocation system changes (P = .004). In other CICUs, the proportion did not significantly change (24.5% [13 of 53 admissions] to 24.1% [20 of 83 admissions]; P = .95). After multivariable adjustment, patients admitted to US transplant centers in the postrevision period were more likely to receive temporary MCS compared with those admitted in the prerevision period (adjusted odds ratio, 2.19; 95% CI, 1.13-4.24; P = .02).
Conclusions and relevance: In the year after implementation of the new UNOS donor heart allocation system, temporary MCS use in patients admitted with ADHF-CS increased in US transplant centers but not in other CICUs. Whether this shift in practice will affect outcomes of patients with ADHF-CS or organ distribution should be evaluated.
Conflict of interest statement
Figures
References
-
- US Department of Health and Human Services, Organ Procurement and Transplantation Network Adult heart allocation. June 15, 2019. Accessed December 1, 2019. https://optn.transplant.hrsa.gov/learn/professional-education/adult-hear...
-
- Morrow DA, Fang JC, Fintel DJ, et al. ; American Heart Association Council on Cardiopulmonary, Critical Care, Perioperative and Resuscitation, Council on Clinical Cardiology, Council on Cardiovascular Nursing, and Council on Quality of Care and Outcomes Research . Evolution of critical care cardiology: transformation of the cardiovascular intensive care unit and the emerging need for new medical staffing and training models: a scientific statement from the American Heart Association. Circulation. 2012;126(11):1408-1428. doi:10.1161/CIR.0b013e31826890b0 - DOI - PubMed
-
- Bohula EA, Katz JN, van Diepen S, et al. ; Critical Care Cardiology Trials Network . Demographics, care patterns, and outcomes of patients admitted to cardiac intensive care units: the Critical Care Cardiology Trials Network prospective North American multicenter registry of cardiac critical illness. JAMA Cardiol. 2019;4(9):928-935. doi:10.1001/jamacardio.2019.2467 - DOI - PMC - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

