Effects of amotosalen treatment on human platelet lysate bioactivity: A proof-of-concept study
- PMID: 32294080
- PMCID: PMC7159197
- DOI: 10.1371/journal.pone.0220163
Effects of amotosalen treatment on human platelet lysate bioactivity: A proof-of-concept study
Abstract
Background: Clinical application of mesenchymal stromal cells (MSCs) usually requires an in vitro expansion step to reach clinically relevant numbers. In vitro cell expansion necessitates supplementation of basal mammalian cell culture medium with growth factors. To avoid using supplements containing animal substances, human platelet lysates (hPL) produced from expired and pathogen inactivated platelet concentrates can be used in place of fetal bovine serum. However, globally, most transfusion units are currently not pathogen inactivated. As blood banks are the sole source of platelet concentrates for hPL production, it is important to ensure product safety and standardized production methods. In this proof-of-concept study we assessed the feasibility of producing hPL from expired platelet concentrates with pathogen inactivation applied after platelet lysis by evaluating the retention of growth factors, cytokines, and the ability to support MSC proliferation and tri-lineage differentiation.
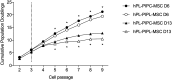
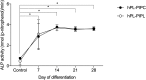
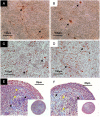
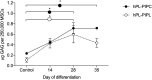
Methodology/principal findings: Bone marrow-derived MSCs (BM-MSCs) were expanded and differentiated using hPL derived from pathogen inactivated platelet lysates (hPL-PIPL), with pathogen inactivation by amotosalen/ultraviolet A treatment applied after lysis of expired platelets. Results were compared to those using hPL produced from conventional expired pathogen inactivated platelet concentrates (hPL-PIPC), with pathogen inactivation applied after blood donation. hPL-PIPL treatment had lower concentrations of soluble growth factors and cytokines than hPL-PIPC treatment. When used as supplementation in cell culture, BM-MSCs proliferated at a reduced rate, but more consistently, in hPL-PIPL than in hPL-PIPC. The ability to support tri-lineage differentiation was comparable between lysates.
Conclusion/significance: These results suggest that functional hPL can be produced from expired and untreated platelet lysates by applying pathogen inactivation after platelet lysis. When carried out post-expiration, pathogen inactivation may provide a valuable solution for further standardizing global hPL production methods, increasing the pool of starting material, and meeting future demand for animal-free supplements in human cell culturing.
Conflict of interest statement
OES and SJM are are shareholders in Platome biotechnology (PB). SJM is the CEO of PB and recived salaries from PB during the study. CC holds no shares and was not paid by BP during the study. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
A double-virally-inactivated (Intercept-solvent/detergent) human platelet lysate for in vitro expansion of human mesenchymal stromal cells.Transfusion. 2019 Jun;59(6):2061-2073. doi: 10.1111/trf.15251. Epub 2019 Mar 25. Transfusion. 2019. PMID: 30912158
-
Expired and Pathogen-Inactivated Platelet Concentrates Support Differentiation and Immunomodulation of Mesenchymal Stromal Cells in Culture.Cell Transplant. 2015;24(8):1545-54. doi: 10.3727/096368914X683043. Epub 2014 Jul 15. Cell Transplant. 2015. PMID: 25198449
-
Platelet lysates produced from expired platelet concentrates support growth and osteogenic differentiation of mesenchymal stem cells.PLoS One. 2013 Jul 11;8(7):e68984. doi: 10.1371/journal.pone.0068984. Print 2013. PLoS One. 2013. PMID: 23874839 Free PMC article.
-
Platelet lysate as a substitute for animal serum for the ex-vivo expansion of mesenchymal stem/stromal cells: present and future.Stem Cell Res Ther. 2016 Jul 13;7(1):93. doi: 10.1186/s13287-016-0352-x. Stem Cell Res Ther. 2016. PMID: 27411942 Free PMC article. Review.
-
Feasibility of Human Platelet Lysate as an Alternative to Foetal Bovine Serum for In Vitro Expansion of Chondrocytes.Int J Mol Sci. 2021 Jan 28;22(3):1269. doi: 10.3390/ijms22031269. Int J Mol Sci. 2021. PMID: 33525349 Free PMC article. Review.
Cited by
-
Human Platelet Lysate for Good Manufacturing Practice-Compliant Cell Production.Int J Mol Sci. 2021 May 13;22(10):5178. doi: 10.3390/ijms22105178. Int J Mol Sci. 2021. PMID: 34068404 Free PMC article. Review.
-
Influence of platelet storage time on human platelet lysates and platelet lysate-expanded mesenchymal stromal cells for bone tissue engineering.Stem Cell Res Ther. 2020 Sep 23;11(1):351. doi: 10.1186/s13287-020-01863-9. Stem Cell Res Ther. 2020. PMID: 32962723 Free PMC article.
-
Expanding applications of allogeneic platelets, platelet lysates, and platelet extracellular vesicles in cell therapy, regenerative medicine, and targeted drug delivery.J Biomed Sci. 2023 Sep 14;30(1):79. doi: 10.1186/s12929-023-00972-w. J Biomed Sci. 2023. PMID: 37704991 Free PMC article. Review.
-
Nanofiltration of growth media supplemented with human platelet lysates for pathogen-safe xeno-free expansion of mesenchymal stromal cells.Cytotherapy. 2020 Aug;22(8):458-472. doi: 10.1016/j.jcyt.2020.04.099. Epub 2020 May 8. Cytotherapy. 2020. PMID: 32536505 Free PMC article.
-
Human platelet lysate standardization across three independent European blood establishments.Stem Cell Res Ther. 2025 Jul 1;16(1):329. doi: 10.1186/s13287-025-04445-9. Stem Cell Res Ther. 2025. PMID: 40598301 Free PMC article.
References
-
- Green L, Allard S, Cardigan R. Modern banking, collection, compatibility testing and storage of blood and blood components. Anaesthesia. 2015;70(Suppl 1):3–9,e2. - PubMed
-
- Bakkour S, Chafets DM, Wen L, Dupuis K, Castro G, Green JM, et al. Assessment of nucleic acid modification induced by amotosalen and ultraviolet A light treatment of platelets and plasma using real-time polymerase chain reaction amplification of variable length fragments of mitochondrial DNA. Transfusion. 2016;56(2):410–20. 10.1111/trf.13360 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

