A refined model of how Yersinia pestis produces a transmissible infection in its flea vector
- PMID: 32294143
- PMCID: PMC7185726
- DOI: 10.1371/journal.ppat.1008440
A refined model of how Yersinia pestis produces a transmissible infection in its flea vector
Abstract
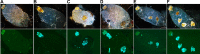
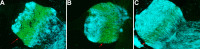
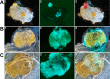
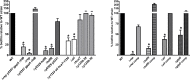
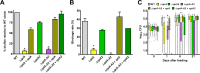
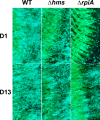
In flea-borne plague, blockage of the flea's foregut by Yersinia pestis hastens transmission to the mammalian host. Based on microscopy observations, we first suggest that flea blockage results from primary infection of the foregut and not from midgut colonization. In this model, flea infection is characterized by the recurrent production of a mass that fills the lumen of the proventriculus and encompasses a large number of Y. pestis. This recurrence phase ends when the proventricular cast is hard enough to block blood ingestion. We further showed that ymt (known to be essential for flea infection) is crucial for cast production, whereas the hmsHFRS operon (known to be essential for the formation of the biofilm that blocks the gut) is needed for cast consolidation. By screening a library of mutants (each lacking a locus previously known to be upregulated in the flea gut) for biofilm formation, we found that rpiA is important for flea blockage but not for colonization of the midgut. This locus may initially be required to resist toxic compounds within the proventricular cast. However, once the bacterium has adapted to the flea, rpiA helps to form the biofilm that consolidates the proventricular cast. Lastly, we used genetic techniques to demonstrate that ribose-5-phosphate isomerase activity (due to the recent gain of a second copy of rpiA (y2892)) accentuated blockage but not midgut colonization. It is noteworthy that rpiA is an ancestral gene, hmsHFRS and rpiA2 were acquired by the recent ancestor of Y. pestis, and ymt was acquired by Y. pestis itself. Our present results (i) highlight the physiopathological and molecular mechanisms leading to flea blockage, (ii) show that the role of a gene like rpiA changes in space and in time during an infection, and (iii) emphasize that evolution is a gradual process punctuated by sudden jumps.
Conflict of interest statement
The authors declare that no competing interests exist.
Figures






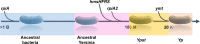
References
-
- Zhou W, Russell CW, Johnson KL, Mortensen RD, Erickson DL. Gene expression analysis of Xenopsylla cheopis (Siphonaptera: Pulicidae) suggests a role for reactive oxygen species in response to Yersinia pestis infection. Journal of medical entomology. 2012;49(2):364–70. 10.1603/me11172 . - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

