Leading a Digital Transformation in the Pharmaceutical Industry: Reimagining the Way We Work in Global Drug Development
- PMID: 32294230
- PMCID: PMC7540662
- DOI: 10.1002/cpt.1850
Leading a Digital Transformation in the Pharmaceutical Industry: Reimagining the Way We Work in Global Drug Development
Abstract
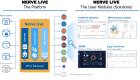
We are experiencing seminal times in computing that seem to define a fourth industrial revolution. This may fundamentally change the way we live, work, and relate to one another. Embracing data and digital information is a top priority for most industries these days, and Life Sciences is no exception. The pharmaceutical industry in particular is fundamentally a data-driven business. Inspired by a desire to "Go Big on Data," we developed a strategic roadmap defining a digital transformation to reimagine the way we work in Novartis Global Drug Development, leveraging data science to generate and inject actionable insights into our best practices. We launched a program called Nerve Live, and built a state-of-the-art data and analytics platform to harness past and present operational data, providing access to decades of drug development "experience" buried across multiple sources. The platform enabled the systematic application of machine learning and predictive analytics to generate "intelligence": new insights across multiple functional areas. To action the insights and create "value," we crafted skillfully designed end-user applications for domain experts to plan, track, predict, compare and monitor domain activities, optimize costs, and maximize quality. Today, the Nerve Live program enables insights-driven decision making at scale, unlocking productivity, and providing transparency across the Novartis Global Drug Development organization and beyond. We identified three main drivers making the Nerve Live program successful and enabling the associated digital transformation to flourish. We discuss the challenges, highlight the benefits, and see the importance of leading the way to become future proof.
© 2020 The Authors. Clinical Pharmacology & Therapeutics published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declared no conflict of interest.
Figures

References
-
- Schwab, K. The fourth industrial revolution – what it means and how to respond. Foreign Affairs <https://www.foreignaffairs.com/articles/2015‐12‐12/fourth‐industrial‐rev...> (2015). Accessed September 30, 2019.
-
- Holst, A. Global smartphone penetration rate as share of population from 2016 to 2020. Statista <https://www.statista.com/statistics/203734/global‐smartphone‐penetration...> (2019). Accessed September 30, 2019.
-
- Jain, S.H. The health care innovation bubble. Healthcare 5, 231–232 (2017). - PubMed
-
- Regulation (EU) 2016/679 of the European Parliament and of the Council of 27 April 2016 on the protection of natural persons with regard to the processing of personal data and on the free movement of such data, and repealing Directive 95/46/EC (General Data Protection Regulation) <http://data.europa.eu/eli/reg/2016/679/oj> (2019).
-
- US Food and Drug Administration . 21 CFR Part 11 <https://gov.ecfr.io/cgi‐bin/text‐idx?SID=a9a7ea74f55b223a7ebc058e54c6c58...>.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

