Transitioning from Preclinical to Clinical Heart Failure with Preserved Ejection Fraction: A Mechanistic Approach
- PMID: 32294958
- PMCID: PMC7230997
- DOI: 10.3390/jcm9041110
Transitioning from Preclinical to Clinical Heart Failure with Preserved Ejection Fraction: A Mechanistic Approach
Abstract
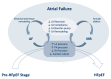
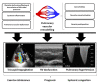
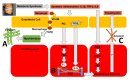
To better understand heart failure with preserved ejection fraction (HFpEF), we need to better characterize the transition from asymptomatic pre-HFpEF to symptomatic HFpEF. The current emphasis on left ventricular diastolic dysfunction must be redirected to microvascular inflammation and endothelial dysfunction that leads to cardiomyocyte remodeling and enhanced interstitial collagen deposition. A pre-HFpEF patient lacks signs or symptoms of heart failure (HF), has preserved left ventricular ejection fraction (LVEF) with incipient structural changes similar to HFpEF, and possesses elevated biomarkers of cardiac dysfunction. The transition from pre-HFpEF to symptomatic HFpEF also involves left atrial failure, pulmonary hypertension and right ventricular dysfunction, and renal failure. This review focuses on the non-left ventricular mechanisms in this transition, involving the atria, right heart cavities, kidneys, and ultimately the currently accepted driver-systemic inflammation. Impaired atrial function may decrease ventricular hemodynamics and significantly increase left atrial and pulmonary pressure, leading to HF symptoms, irrespective of left ventricle (LV) systolic function. Pulmonary hypertension and low right-ventricular function are associated with the incidence of HF. Interstitial fibrosis in the heart, large arteries, and kidneys is key to the pathophysiology of the cardiorenal syndrome continuum. By understanding each of these processes, we may be able to halt disease progression and eventually extend the time a patient remains in the asymptomatic pre-HFpEF stage.
Keywords: atrial failure; heart failure with preserved ejection fraction; inflammation; pulmonary artery; renal function; right ventricle.
Conflict of interest statement
Dr. Bayes-Genis reports grants and personal fees from AstrsaZeneca, Vifor, Novartis, Roche Diagnostics, and Critical diagnostics. Dr. NUNEZ reports grants and personal fees from Astra Zeneca, grants and personal fees from Vifor Pharma, personal fees from Novartis, personal fees from Beohringher Ingelheim, personal fees from Rovi, and personal fees from Novo Nordisk outside the submitted work. Dr. Rossignol reports grants and personal fees from AstraZeneca, Bayer, CVRx, personal fees from Fresenius, grants and personal fees from Novartis, personal fees from Grunenthal, Servier, Stealth Peptides, Vifor Fresenius Medical Care Renal Pharma, Idorsia, Novo Nordisk, Ablative Solutions, G3P, Corvidia, and Relypsa outside of the submitted work and is a cofounder of CardioRenal, a company developing a telemonitoring loop for heart failure (including hemoglobin, potassium, and creatinine monitoring).
Figures


References
-
- Lee D.S., Gona P., Vasan R.S., Larson M.G., Benjamin E.J., Wang T.J., Tu J.V., Levy D. Relation of disease pathogenesis and risk factors to heart failure with preserved or reduced ejection fraction: Insights from the Framingham Heart Study of the National Heart, Lung, and Blood Institute. Circulation. 2009;119:3070–3077. doi: 10.1161/CIRCULATIONAHA.108.815944. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

