Sink/Source Balance of Leaves Influences Amino Acid Pools and Their Associated Metabolic Fluxes in Winter Oilseed Rape (Brassica napus L.)
- PMID: 32295054
- PMCID: PMC7240945
- DOI: 10.3390/metabo10040150
Sink/Source Balance of Leaves Influences Amino Acid Pools and Their Associated Metabolic Fluxes in Winter Oilseed Rape (Brassica napus L.)
Abstract
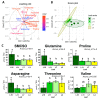
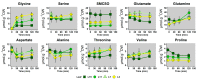
Nitrogen remobilization processes from source to sink tissues in plants are determinant for seed yield and their implementation results in a complete reorganization of the primary metabolism during sink/source transition. Here, we decided to characterize the impact of the sink/source balance on amino acid metabolism in the leaves of winter oilseed rape grown at the vegetative stage. We combined a quantitative metabolomics approach with an instationary 15N-labeling experiment by using [15N]L-glycine as a metabolic probe on leaf ranks with a gradual increase in their source status. We showed that the acquisition of the source status by leaves was specifically accompanied by a decrease in asparagine, glutamine, proline and S-methyl-l-cysteine sulphoxide contents and an increase in valine and threonine contents. Dynamic analysis of 15N enrichment and concentration of amino acids revealed gradual changes in the dynamics of amino acid metabolism with respect to the sink/source status of leaf ranks. Notably, nitrogen assimilation into valine, threonine and proline were all decreased in source leaves compared to sink leaves. Overall, our results suggested a reduction in de novo amino acid biosynthesis during sink/source transition at the vegetative stage.
Keywords: Brassica napus; SMCSO; amino acid metabolism; isotopically non-stationary steady-state metabolic flux analysis; metabolomics; proline; senescence; sink/source balance; threonine; valine; winter oilseed rape.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study, in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures



References
-
- Malagoli P., Laine P., Rossato L., Ourry A. Dynamics of nitrogen uptake and mobilization in field-grown winter oilseed rape (Brassica napus) from stem extension to harvest. Ii. An 15n-labelling-based simulation model of n partitioning between vegetative and reproductive tissues. Ann. Bot. 2005;95:1187–1198. doi: 10.1093/aob/mci131. - DOI - PMC - PubMed
-
- Rathke G.W., Christen O., Diepenbrock W. Effects of nitrogen source and rate on productivity and quality of winter oilseed rape (Brassica napus L.) grown in different crop rotations. Field Crop. Res. 2005;94:103–113. doi: 10.1016/j.fcr.2004.11.010. - DOI
Grants and funding
LinkOut - more resources
Full Text Sources

