Proteomic Characterisation of Lupin (Lupinus angustifolius) Milk as Influenced by Extraction Techniques, Seed Coat and Cultivars
- PMID: 32295067
- PMCID: PMC7221801
- DOI: 10.3390/molecules25081782
Proteomic Characterisation of Lupin (Lupinus angustifolius) Milk as Influenced by Extraction Techniques, Seed Coat and Cultivars
Abstract
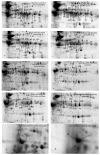
Lupin seeds are rich in proteins and other essential ingredients that can help to improve human health. The protein contents in both whole and split seeds of two lupin cultivars (Mandleup and PBA Jurien) were used to produce the lupin milk using the cheesecloth and centrifuge method. Proteins were extracted from the lupin milk using thiourea/urea solubilization. The proteins were separated by a two-dimensional polyacrylamide gel electrophoresis and then identified with mass spectrometry. A total of 230 protein spots were identified, 60 of which showed differential abundances. The cheesecloth separation showed protein extractability much better than that of the centrifuge method for both the cultivars. The results from this study could offer guidance for future comparative analysis and identification of lupin milk protein and provide effective separation technique to determine specific proteins in the cheese-making process.
Keywords: Lupin; Mandelup; PBA Jurien; centrifuge separation; cheesecloth separation; protein; two-dimensional gel electrophoresis.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Chew P.G., Casey A.J., Johnson S.K. Protein quality and physico-functionality of Australian sweet lupin (Lupinus angustifolius cv. Gungurru) protein concentrates prepared by isoelectric precipitation or ultrafiltration. Food Chem. 2003;83:575–583. doi: 10.1016/S0308-8146(03)00156-0. - DOI
-
- Johnson S.K., McQuillan P.L., Sin J.H., Ball M.J. Sensory acceptability of white bread with added Australian sweet lupin (Lupinus angustifolius) kernel fibre and its glycaemic and insulinaemic responses when eaten as a breakfast. J. Sci. Food Agric. 2003;83:1366–1372. doi: 10.1002/jsfa.1552. - DOI
-
- Martínez-Villaluenga C., Zieliński H., Frias J., Piskuła M.K., Kozłowska H., Vidal-Valverde C. Antioxidant capacity and polyphenolic content of high-protein lupin products. Food Chem. 2009;112:84–88. doi: 10.1016/j.foodchem.2008.05.040. - DOI
-
- Jayasena V., Chih H.J., Nasar-Abbas S. Functional properties of sweet lupin protein isolated and tested at various pH levels. [(accessed on 19 March 2020)];Res. J. Agric. Biol. Sci. 2010 6:130–137. Available online: http://hdl.handle.net/20.500.11937/19417.
-
- Kiosseoglou A., Doxastakis G., Alevisopoulos S., Kasapis S. Physical characterization of thermally induced networks of lupin protein isolates prepared by isoelectric precipitation and dialysis. Int. J. Food Sci. Technol. 1999;34:253–263. doi: 10.1046/j.1365-2621.1999.00260.x. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

