Characterization and Mathematical Modeling of Alginate/Chitosan-Based Nanoparticles Releasing the Chemokine CXCL12 to Attract Glioblastoma Cells
- PMID: 32295255
- PMCID: PMC7238026
- DOI: 10.3390/pharmaceutics12040356
Characterization and Mathematical Modeling of Alginate/Chitosan-Based Nanoparticles Releasing the Chemokine CXCL12 to Attract Glioblastoma Cells
Erratum in
-
Erratum: Gascon S.; et al. Characterization and Mathematical Modeling of Alginate/Chitosan-Based Nanoparticles Releasing the Chemokine CXCL12 to Attract Glioblastoma Cells. Pharmaceutics 2020, 12, 356.Pharmaceutics. 2020 Nov 27;12(12):1153. doi: 10.3390/pharmaceutics12121153. Pharmaceutics. 2020. PMID: 33261225 Free PMC article.
Abstract
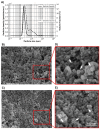
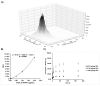
Chitosan (Chit) currently used to prepare nanoparticles (NPs) for brain application can be complexed with negatively charged polymers such as alginate (Alg) to better entrap positively charged molecules such as CXCL12. A sustained CXCL12 gradient created by a delivery system can be used, as a therapeutic approach, to control the migration of cancerous cells infiltrated in peri-tumoral tissues similar to those of glioblastoma multiforme (GBM). For this purpose, we prepared Alg/Chit NPs entrapping CXCL12 and characterized them. We demonstrated that Alg/Chit NPs, with an average size of ~250 nm, entrapped CXCL12 with ~98% efficiency for initial mass loadings varying from 0.372 to 1.490 µg/mg NPs. The release kinetic profiles of CXCL12 were dependent on the initial mass loading, and the released chemokine from NPs after seven days reached 12.6%, 32.3%, and 59.9% of cumulative release for initial contents of 0.372, 0.744, and 1.490 µg CXCL12/mg NPs, respectively. Mathematical modeling of released kinetics showed a predominant diffusive process with strong interactions between Alg and CXCL12. The CXCL12-NPs were not toxic and did not promote F98 GBM cell proliferation, while the released CXCL12 kept its chemotaxis effect. Thus, we developed an efficient and tunable CXCL12 delivery system as a promising therapeutic strategy that aims to be injected into a hydrogel used to fill the cavity after surgical tumor resection. This system will be used to attract infiltrated GBM cells prior to their elimination by conventional treatment without affecting a large zone of healthy brain tissue.
Keywords: alginate; cell migration; chemokine; chitosan; delivery system; mathematical modeling; nanoparticles.
Conflict of interest statement
The authors declare no conflict of interest.
Figures






References
-
- Elkadery A.A.S., Elsherif E.A., Ezz Eldin H.M., Fahmy I.A.F., Mohammad O.S. Efficient therapeutic effect of Nigella sativa aqueous extract and chitosan nanoparticles against experimentally induced Acanthamoeba keratitis. Parasitol. Res. 2019;118:2443–2454. doi: 10.1007/s00436-019-06359-x. - DOI - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

