Selected Pharmaceuticals in Different Aquatic Compartments: Part II-Toxicity and Environmental Risk Assessment
- PMID: 32295269
- PMCID: PMC7221825
- DOI: 10.3390/molecules25081796
Selected Pharmaceuticals in Different Aquatic Compartments: Part II-Toxicity and Environmental Risk Assessment
Abstract
Potential risks associated with releases of human pharmaceuticals into the environment have become an increasingly important issue in environmental health. This concern has been driven by the widespread detection of pharmaceuticals in all aquatic compartments. Therefore, 22 pharmaceuticals, 6 metabolites and transformation products, belonging to 7 therapeutic groups, were selected to perform a review on their toxicity and environmental risk assessment (ERA) in different aquatic compartments, important issues to tackle the water framework directive (WFD). The toxicity data collected reported, with the exception of anxiolytics, at least one toxicity value for concentrations below 1 µg L-1. The results obtained for the ERA revealed risk quotients (RQs) higher than 1 in all the aquatic bodies and for the three trophic levels, algae, invertebrates and fish, posing ecotoxicological pressure in all of these compartments. The therapeutic groups with higher RQs were hormones, antiepileptics, anti-inflammatories and antibiotics. Unsurprisingly, RQs values were highest in wastewaters, however, less contaminated water bodies such as groundwaters still presented maximum values up to 91,150 regarding 17α-ethinylestradiol in fish. Overall, these results present an important input for setting prioritizing measures and sustainable strategies, minimizing their impact in the aquatic environment.
Keywords: aquatic compartments; environmental contaminants; environmental risk assessment; pharmaceuticals; pharmaceuticals toxicity.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
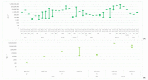
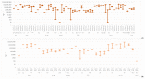


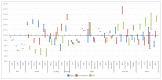


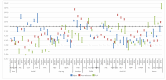
References
-
- Robles-Molina J., Lara-Ortega F.J., Gilbert-López B., García-Reyes J.F., Molina-Díaz A. Multi-residue method for the determination of over 400 priority and emerging pollutants in water and wastewater by solid-phase extraction and liquid chromatography-time-of-flight mass spectrometry. J. Chromatogr. A. 2014;1350:30–43. doi: 10.1016/j.chroma.2014.05.003. - DOI - PubMed
-
- European Medicines Agency Guideline on the Environmental Risk Assessment of Medicinal Products for Human Use. European Medicines Agency; London, UK: 2006.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

