Genotypes and Phenotypes: A Search for Influential Genes in Diabetic Retinopathy
- PMID: 32295293
- PMCID: PMC7215289
- DOI: 10.3390/ijms21082712
Genotypes and Phenotypes: A Search for Influential Genes in Diabetic Retinopathy
Abstract
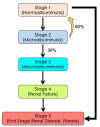
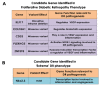
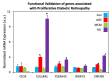
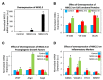
Although gene-environment interactions are known to play an important role in the inheritance of complex traits, it is still unknown how a genotype and the environmental factors result in an observable phenotype. Understanding this complex interaction in the pathogenesis of diabetic retinopathy (DR) remains a big challenge as DR appears to be a disease with heterogenous phenotypes with multifactorial influence. In this review, we examine the natural history and risk factors related to DR, emphasizing distinct clinical phenotypes and their natural course in retinopathy. Although there is strong evidence that duration of diabetes and metabolic factors play a key role in the pathogenesis of DR, accumulating new clinical studies reveal that this disease can develop independently of duration of diabetes and metabolic dysfunction. More recently, studies have emphasized the role of genetic factors in DR. However, linkage analyses, candidate gene studies, and genome-wide association studies (GWAS) have not produced any statistically significant results. Our recently initiated genomics study, the Diabetic Retinopathy Genomics (DRGen) Study, aims to examine the contribution of rare and common variants in the development DR, and how they can contribute to clinical phenotype, rate of progression, and response to available therapies. Our preliminary findings reveal a novel set of genetic variants associated with proangiogenic and inflammatory pathways that may contribute to DR pathogenesis. Further investigation of these variants is necessary and may lead to development of novel biomarkers and new therapeutic targets in DR.
Keywords: diabetes; diabetic retinopathy; genomics; genotype; phenotype.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Paxton W.A., Martin S.R., Tse D., O’Brien T.R., Skurnick J., VanDevanter N.L., Padian N., Braun J.F., Kotler D.P., Wolinsky S.M., et al. Relative resistance to HIV-1 infection of CD4 lymphocytes from persons who remain uninfected despite multiple high-risk sexual exposure. Nat. Med. 1996;2:412–417. doi: 10.1038/nm0496-412. - DOI - PubMed
-
- Liu R., Paxton W.A., Choe S., Ceradini D., Martin S.R., Horuk R., MacDonald M.E., Stuhlmann H., Koup R.A., Landau N.R. Homozygous defect in HIV-1 coreceptor accounts for resistance of some multiply-exposed individuals to HIV-1 infection. Cell. 1996;86:367–377. doi: 10.1016/S0092-8674(00)80110-5. - DOI - PubMed
-
- Dobzhansky T. Genetics and the Origin of Species. 1st ed. Columbia University Press; New York, NY, USA: 1937.
-
- Dobzhansky T. Genetics and the Origin of Species. 3rd ed. Columbia University Press; New York, NY, USA: 1951.
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

