The Plasma Membrane-An Integrating Compartment for Mechano-Signaling
- PMID: 32295309
- PMCID: PMC7238056
- DOI: 10.3390/plants9040505
The Plasma Membrane-An Integrating Compartment for Mechano-Signaling
Abstract
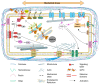
Plants are able to sense their mechanical environment. This mechanical signal is used by the plant to determine its phenotypic features. This is true also at a smaller scale. Morphogenesis, both at the cell and tissue level, involves mechanical signals that influence specific patterns of gene expression and trigger signaling pathways. How a mechanical stress is perceived and how this signal is transduced into the cell remains a challenging question in the plant community. Among the structural components of plant cells, the plasma membrane has received very little attention. Yet, its position at the interface between the cell wall and the interior of the cell makes it a key factor at the nexus between biochemical and mechanical cues. So far, most of the key players that are described to perceive and maintain mechanical cell status and to respond to a mechanical stress are localized at or close to the plasma membrane. In this review, we will focus on the importance of the plasma membrane in mechano-sensing and try to illustrate how the composition of this dynamic compartment is involved in the regulatory processes of a cell to respond to mechanical stress.
Keywords: Arabidopsis; cell wall integrity; mechanical stress; mechanosensitive signaling pathways; plasma membrane; receptor-like kinase.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources

