Expression Profiling, Downstream Signaling, and Inter-subunit Interactions of GPA2/GPB5 in the Adult Mosquito Aedes aegypti
- PMID: 32296389
- PMCID: PMC7137729
- DOI: 10.3389/fendo.2020.00158
Expression Profiling, Downstream Signaling, and Inter-subunit Interactions of GPA2/GPB5 in the Adult Mosquito Aedes aegypti
Abstract
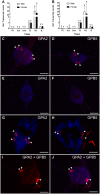
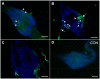
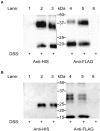
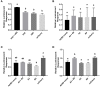
GPA2/GPB5 and its receptor constitute a glycoprotein hormone-signaling system native to the genomes of most vertebrate and invertebrate organisms. Unlike the well-studied gonadotropins and thyrotropin, the exact function of GPA2/GPB5 remains elusive, and whether it elicits its functions as heterodimers, homodimers or as independent monomers remains unclear. Here, the glycoprotein hormone signaling system was investigated in adult mosquitoes, where GPA2 and GPB5 subunit expression was mapped and modes of its signaling were characterized. In adult Aedes aegypti mosquitoes, GPA2 and GPB5 transcripts co-localized to bilateral pairs of neuroendocrine cells, positioned within the first five abdominal ganglia of the central nervous system. Unlike GPA2/GPB5 homologs in human and fly, GPA2/GPB5 subunits in A. aegypti lacked evidence of heterodimerization. Rather, cross-linking analysis to determine subunit interactions revealed A. aegypti GPA2 and GPB5 subunits may form homodimers, although treatments with independent subunits did not demonstrate receptor activity. Since mosquito GPA2/GPB5 heterodimers were not evident by heterologous expression, a tethered fusion construct was generated for expression of the subunits as a single polypeptide chain to mimic heterodimer formation. Our findings revealed A. aegypti LGR1 elicited constitutive activity with elevated levels of cAMP. However, upon treatment with recombinant tethered GPA2/GPB5, an inhibitory G protein (Gi/o) signaling cascade is initiated and forskolin-induced cAMP production is inhibited. These results further support the notion that heterodimerization is a requirement for glycoprotein hormone receptor activation and provide novel insight to how signaling is achieved for GPA2/GPB5, an evolutionary ancient neurohormone.
Keywords: GPA2/GPB5; glycoprotein hormone; heterodimer; homodimer; leucine-rich repeat-containing G protein coupled-receptor 1; mosquito; thyrostimulin.
Copyright © 2020 Rocco and Paluzzi.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

