Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response
- PMID: 32296651
- PMCID: PMC7136410
- DOI: 10.3389/fcimb.2020.00130
Cytomegalovirus Latency and Reactivation: An Intricate Interplay With the Host Immune Response
Abstract
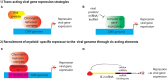
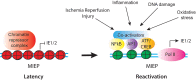
CMV is an ancient herpesvirus that has co-evolved with its host over millions of years. The 236 kbp genome encodes at least 165 genes, four non-coding RNAs and 14 miRNAs. Of the protein-coding genes, 43-44 are core replication genes common to all herpesviruses, while ~30 are unique to betaherpesviruses. Many CMV genes are involved in evading detection by the host immune response, and others have roles in cell tropism. CMV replicates systemically, and thus, has adapted to various biological niches within the host. Different biological niches may place competing demands on the virus, such that genes that are favorable in some contexts are unfavorable in others. The outcome of infection is dependent on the cell type. In fibroblasts, the virus replicates lytically to produce infectious virus. In other cell types, such as myeloid progenitor cells, there is an initial burst of lytic gene expression, which is subsequently silenced through epigenetic repression, leading to establishment of latency. Latently infected monocytes disseminate the virus to various organs. Latency is established through cell type specific mechanisms of transcriptional silencing. In contrast, reactivation is triggered through pathways activated by inflammation, infection, and injury that are common to many cell types, as well as differentiation of myeloid cells to dendritic cells. Thus, CMV has evolved a complex relationship with the host immune response, in which it exploits cell type specific mechanisms of gene regulation to establish latency and to disseminate infection systemically, and also uses the inflammatory response to infection as an early warning system which allows the virus to escape from situations in which its survival is threatened, either by cellular damage or infection of the host with another pathogen. Spontaneous reactivation induced by cellular aging/damage may explain why extensive expression of lytic genes has been observed in recent studies using highly sensitive transcriptome analyses of cells from latently infected individuals. Recent studies with animal models highlight the potential for harnessing the host immune response to blunt cellular injury induced by organ transplantation, and thus, prevent reactivation of CMV and its sequelae.
Keywords: cytomegalovirus; epigenetics; inflammation; latency; oxidative stress; reactivation.
Copyright © 2020 Forte, Zhang, Thorp and Hummel.
Figures
References
-
- Aird W. C. (2007b). Phenotypic heterogeneity of the endothelium: II. Representative vascular beds. Circ. Res. 100, 174–190. 10.1161/01.RES.0000255690.03436.ae - DOI - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

