Perspective: Implications of Ligand-Receptor Binding Kinetics for Therapeutic Targeting of G Protein-Coupled Receptors
- PMID: 32296761
- PMCID: PMC7155193
- DOI: 10.1021/acsptsci.0c00012
Perspective: Implications of Ligand-Receptor Binding Kinetics for Therapeutic Targeting of G Protein-Coupled Receptors
Abstract
The concept of ligand-receptor binding kinetics has been broadly applied in drug development pipelines focusing on G protein-coupled receptors (GPCRs). The ligand residence time (RT) for a receptor describes how long a ligand-receptor complex exists, and is defined as the reciprocal of the dissociation rate constant (k off). RT has turned out to be a valuable parameter for GPCR researchers focusing on drug development as a good predictor of in vivo efficacy. The positive correlation between RT and in vivo efficacy has been established for several drugs targeting class A GPCRs (e.g., the neurokinin-1 receptor (NK1R), the β2 adrenergic receptor (β2AR), and the muscarinic 3 receptor (M3R)) and for drugs targeting class B1 (e.g., the glucagon-like peptide 1 receptor (GLP-1R)). Recently, the association rate constant (k on) has gained similar attention as another parameter affecting in vivo efficacy. In the current perspective, we address the importance of studying ligand-receptor binding kinetics for therapeutic targeting of GPCRs, with an emphasis on how binding kinetics can be altered by subtle molecular changes in the ligands and/or the receptors and how such changes affect treatment outcome. Moreover, we speculate on the impact of binding kinetic parameters for functional selectivity and sustained receptor signaling from endosomal compartments; phenomena that have gained increasing interest in attempts to improve therapeutic targeting of GPCRs.
Copyright © 2020 American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
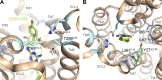




References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
