In vitro modeling of glioblastoma initiation using PDGF-AA and p53-null neural progenitors
- PMID: 32296841
- PMCID: PMC7594559
- DOI: 10.1093/neuonc/noaa093
In vitro modeling of glioblastoma initiation using PDGF-AA and p53-null neural progenitors
Abstract
Background: Imagining ways to prevent or treat glioblastoma (GBM) has been hindered by a lack of understanding of its pathogenesis. Although overexpression of platelet derived growth factor with two A-chains (PDGF-AA) may be an early event, critical details of the core biology of GBM are lacking. For example, existing PDGF-driven models replicate its microscopic appearance, but not its genomic architecture. Here we report a model that overcomes this barrier to authenticity.
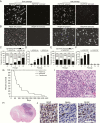
Methods: Using a method developed to establish neural stem cell cultures, we investigated the effects of PDGF-AA on subventricular zone (SVZ) cells, one of the putative cells of origin of GBM. We microdissected SVZ tissue from p53-null and wild-type adult mice, cultured cells in media supplemented with PDGF-AA, and assessed cell viability, proliferation, genome stability, and tumorigenicity.
Results: Counterintuitive to its canonical role as a growth factor, we observed abrupt and massive cell death in PDGF-AA: wild-type cells did not survive, whereas a small fraction of null cells evaded apoptosis. Surviving null cells displayed attenuated proliferation accompanied by whole chromosome gains and losses. After approximately 100 days in PDGF-AA, cells suddenly proliferated rapidly, acquired growth factor independence, and became tumorigenic in immune-competent mice. Transformed cells had an oligodendrocyte precursor-like lineage marker profile, were resistant to platelet derived growth factor receptor alpha inhibition, and harbored highly abnormal karyotypes similar to human GBM.
Conclusion: This model associates genome instability in neural progenitor cells with chronic exposure to PDGF-AA and is the first to approximate the genomic landscape of human GBM and the first in which the earliest phases of the disease can be studied directly.
Keywords: GBM; PDGF-AA; genome instability; glioblastoma; model; p53.
© The Author(s) 2020. Published by Oxford University Press on behalf of the Society for Neuro-Oncology. All rights reserved. For permissions, please e-mail: journals.permissions@oup.com.
Figures



References
-
- Korber V, Yang J, Barah P, et al. . Evolutionary trajectories of IDH(wt) glioblastomas reveal a common path of early tumorigenesis instigated years ahead of initial diagnosis. Cancer Cell. 2019; 35(4):692–704 e612. - PubMed
-
- Koga T, Benitez JA, Chaim IA, et al. . Glioblastomas derived from genetically modified pluripotent stem cells recapitulate pathobiology. BioRxiv. 2019. doi: 10.1101/576009. - DOI
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

